Abstract
Background
Herpes zoster develops via reactivation of the latent varicella zoster virus (VZV) in neuronal ganglia as host immunity declines. In Korea, seroprevalence of VZV is very high and population at risk for herpes zoster is increasing. The goal of this study is to evaluate the infection rate of the VZV and the lifetime prevalence of herpes zoster, and to determine the genotype of VZV.
Materials and Methods
Serum IgG antibody titer was measured in 399 patients. Lifetime prevalence of herpes zoster was evaluated through a survey of 2,054 participants. VZV was isolated by cell culture technique using MRC-5 cells. To determine the genotype of VZV, ORF 22, 38, 54, 62 were amplified by PCR, and after digestion of the PCR products with enzymes pstI, bglI, and smaI, restriction-fragment-length-polymorphism (RFLP) was analysed. The amplified ORF 22 PCR product was sequenced and checked for single nucleotide polymorphisms.
Results
The overall seroprevalence of VZV IgG in adults was 93.9% (375/399). The overall lifetime prevalence of herpes zoster was 13.7% (282/2,054). Of the patients with herpes zoster, 17.7% (50/282) of patients experienced postherpetic neuralgia for more than one month. All 22 VZV isolates were of J genotype; 21 (95.4%) isolates were all pstI+ bglI+ smaI-, and 1 (4.5%) isolate was pstI- bglI+ smaI- (pOka) genotype.
Figures and Tables
Figure 1
RFLP Pattern of ORF 38, 54, and 62 Product Digested with pstI, bglI, smaI. A) A 350bp ORF 38 product was digested with pstI. The product yielded 2 fragments of 250bp and 100bp in size. B) Digestion of 268bp ORF 62 product with smaI yielded 3 fragments 153bp, 79bp, 36bp in size. C) Digestion of 222bp ORF 54 product with bglI yielded 2 fragments 137bp and 87bp in size.
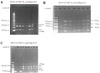
Table 4
Prevalence and Duration of Postherpetic Neuralgia in 282 Patients with a History of Herpes Zoster

References
2. Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos L, De Serres G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001. 27:305–314.

3. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965. 58:9–20.

4. Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995. 155:1605–1609.

5. Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000. 342:635–645.

6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002. 347:340–346.
7. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004. 4:26–33.

8. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975. 25:571–575.
9. Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996. 335:32–42.

10. Population census 2005. Age over 60 years, City and province statistics. KOSIS. Available at: http://www.kosis.kr.Accessed23October2007.
11. Park SY, Kim JY, Kim CD, Kim CW, Lee KS. A clinical study on herpes zoster during the last 10-year-period (1994-2003). Korean J Dermatol. 2004. 42:1531–1535.
12. Kang CI, Choi CM, Hong SS, Kim HB, Kim NJ, Oh MD, Choe KW. The incidence of herpes zoster in otherwise healthy young soldiers of korean army. Infect Chemother. 2006. 38:45–46.
13. Kim SY, Cho BH, Kim JH. A 5 - Year Clinical Study on Herpes Zoster: 1990 - 1994. Korean J Dermatol. 1997. 35:266–272.
14. Shin DY, Koo DW. Statistical analysis of herpes zoster in chuncheon and the northern kangwon province ( 1994-1996 ). Korean J Dermatol. 1998. 36:422–429.
15. Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis. 2005. 191:2002–2007.

16. Adams SG, Dohner DE, Gelb LD. Restriction fragment differences between the genomes of the Oka varicella vaccine virus and American wild-type varicella-zoster virus. J Med Virol. 1989. 29:38–45.

17. Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, Seward JF. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health. 2005. 5:68.

18. LaRussa P, Lungu O, Hardy I, Gershon A, Steinberg SP, Silverstein S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J Virol. 1992. 66:1016–1020.

19. Loparev VN, Gonzalez A, Deleon-Carnes M, Tipples G, Fickenscher H, Torfason EG, Schmid DS. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol. 2004. 78:8349–8358.

20. Kilgore PE, Kruszon-Moran D, Seward JF, Jumaan A, Van Loon FP, Forghani B, McQuillan GM, Wharton M, Fehrs LJ, Cossen CK, Hadler SC. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003. 70:Suppl 1. S111–S118.

21. Dayan GH, Panero MS, Debbag R, Urquiza A, Molina M, Prieto S, Del Carmen Perego M, Scagliotti G, Galimberti D, Carroli G, Wolff C, Schmid DS, Loparev V, Guris D, Seward J. Varicella seroprevalence and molecular epidemiology of varicella-zoster virus in Argentina, 2002. J Clin Microbiol. 2004. 42:5698–5704.

22. Choi HJ, Shim YS, Jeong SY. Susceptibility of health care workers to measles, rubella, and varicella at a university hospital. Infect Chemother. 2003. 35:401–406.
23. Kim OJ, Kim SS, Choi BS, Suh SD, Lee MW, Kim KS, Park MS, Lee JS. Consistency of the low seroprevalence of human herpesvirus 8 and the rarity of kaposi`s sarcoma in south Korea. J Bacteriol Virol. 2001. 31:275–279.
24. Shin HS, Oh HS, Kim SM, Joong KN, Choi HJ, Oh MD, Lee HJ, Choe KW. Prevalence of measles, rubella and varicellazoster antibodies in hospital personnel. Korean J Infect Dis. 1997. 29:29–32.
26. Arvin AM. Humoral and cellular immunity to varicella-zoster virus: an overview. J Infect Dis. 2008. 197:Suppl 2. S58–S60.

27. Schmader K, Gnann JW Jr, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis. 2008. 197:Suppl 2. S207–S215.

28. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007. 82:1341–1349.

29. Dworkin RH, Schmader KE. Treatment and prevention of postherpetic neuralgia. Clin Infect Dis. 2003. 36:877–882.

30. Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986. 67:1759–1816.

31. Sauerbrei A, Wutzler P. Different genotype pattern of varicella-zoster virus obtained from patients with varicella and zoster in Germany. J Med Virol. 2007. 79:1025–1031.

32. Muir WB, Nichols R, Breuer J. Phylogenetic analysis of varicella-zoster virus: evidence of intercontinental spread of genotypes and recombination. J Virol. 2002. 76:1971–1979.

33. Sengupta N, Taha Y, Scott FT, Leedham-Green ME, Quinlivan M, Breuer J. Varicella-zoster-virus genotypes in East London: a prospective study in patients with herpes zoster. J Infect Dis. 2007. 196:1014–1020.

34. Loparev VN, Rubtcova EN, Bostik V, Govil D, Birch CJ, Druce JD, Schmid DS, Croxson MC. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J Virol. 2007. 81:12758–12765.

35. Loparev V, Martro E, Rubtcova E, Rodrigo C, Piette JC, Caumes E, Vernant JP, Schmid DS, Fillet AM. Toward universal varicella-zoster virus (VZV) genotyping: diversity of VZV strains from France and Spain. J Clin Microbiol. 2007. 45:559–563.

36. Sauerbrei A, Zell R, Philipps A, Wutzler P. Genotypes of varicella-zoster virus wild-type strains in Germany. J Med Virol. 2008. 80:1123–1130.

37. Takayama M, Takayama N, Inoue N, Kameoka Y. Application of long PCR method of identification of variations in nucleotide sequences among varicella-zoster virus isolates. J Clin Microbiol. 1996. 34:2869–2874.

38. Argaw T, Cohen JI, Klutch M, Lekstrom K, Yoshikawa T, Asano Y, Krause PR. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J Infect Dis. 2000. 181:1153–1157.

39. Gomi Y, Sunamachi H, Mori Y, Nagaike K, Takahashi M, Yamanishi K. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J Virol. 2002. 76:11447–11459.

40. Loparev VN, Argaw T, Krause PR, Takayama M, Schmid DS. Improved identification and differentiation of varicella-zoster virus (VZV) wild-type strains and an attenuated varicella vaccine strain using a VZV open reading frame 62-based PCR. J Clin Microbiol. 2000. 38:3156–3160.

41. Quinlivan M, Hawrami K, Barrett-Muir W, Aaby P, Arvin A, Chow VT, John TJ, Matondo P, Peiris M, Poulsen A, Siqueira M, Takahashi M, Talukder Y, Yamanishi K, Leedham-Green M, Scott FT, Thomas SL, Breuer J. The molecular epidemiology of varicella-zoster virus: evidence for geographic segregation. J Infect Dis. 2002. 186:888–894.

42. Barrett-Muir W, Scott FT, Aaby P, John J, Matondo P, Chaudhry QL, Siqueira M, Poulsen A, Yaminishi K, Breuer J. Genetic variation of varicella-zoster virus: evidence for geographical separation of strains. J Med Virol. 2003. 70:Suppl 1. S42–S47.

43. Faga B, Maury W, Bruckner DA, Grose C. Identification and mapping of single nucleotide polymorphisms in the varicellazoster virus genome. Virology. 2001. 280:1–6.

44. Park HK, Seoh JY. Antigen analysis and restriction fragment length polymorphysm of the polymerase chain reaction products of Varicella-Zoster virus wild strains isolated in Korea. J Korean Soc Microbiol. 1997. 32:265–274.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download