Abstract
Pituitary hyperplasia associated with untreated primary hypothyroidism in children is a rare condition. There are only a few reports on this condition in children, and especially when pituitary hyperplasia is accompanied with Hashimoto thyroiditis and growth arrest. Here, we describe an unusual association of pituitary hyperplasia with hypothyroidism and growth retardation, and this was all caused by Hashimoto thyroiditis. Hormonal testing showed a low thyroxine level and a high thyroid stimulating hormone level, elevated anti-thyroglobulin, low growth hormone levels and prepubertal levels of gonadotropins. A large intrasellar mass expanding beyond the sella turcica was detected on magnetic resonance imaging (MRI). Homogeneous contrast enhancement of mass highly suggested that it was a pituitary hyperplasia rather than a pituitary tumor. Therapy with L-thyroxine resulted in rapid improvement of the clinical signs, including renewed growth, normalization of the hormone levels and resolution of the pituitary hyperplasia on MRI within 90 days. In children, prolonged unrecognized primary hypothyroidism might be accompanied by growth deficiency and pubertal disharmony. Physicians must be aware of pituitary hyperplasia in these cases.
The differential diagnosis of nonpituitary sellar masses in children is broad. It includes germ cell tumors, gliomas, meningiomas, metastatic tumors, vascular lesions, and granulomatous, infectious, and inflammatory processes in the intrasellar area. Furthermore, the physiologic increase in the size of pituitary gland during puberty may be observed causing confusion with pituitary adenoma. Although boys may have enlargement of pituitary glands, typically, these are less than 7 to 8 mm[1]. Even though it rarely happened, primary hypothyroidism may be complicated by an enlarged pituitary gland, particularly in children. Earlier studies showed long-standing untreated hypothyroidism may result in enlargement of pituitary glands. The subsequent regression of glands with thyroxine therapy indicated pituitary hyperplasia rather than adenoma or other cause of sellar masses. This should be clinically apparent and resolved within a few months of treatment[2,3]. However, neurosurgery for a presumed primary pituitary tumor has been unnecessarily performed in cases of secondary pituitary enlargement in adults[4] and also in children as recently reported[5].
Our observation emphasizes the importance of diagnosing and treating primary hypothyroidism prior to considering surgery for confirmation of possible pituitary adenoma, especially in children. A brief review of related literatures was also made.
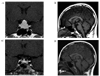
A 15-year-old boy presented to his local hospital with complaints of short stature, weight gain and chronic fatigue. He was diagnosed with hypothyroidism when he was found to have a thyroid stimulating hormone (TSH) of 50 mIU/mL, and free T4 of 0.64 pmol/L, 1 month before. As the finding by brain computed tomography (CT) was suggestive of a pituitary tumor, he was referred for further neurosurgical evaluation. Sellar magnetic resonance imaging (MRI) revealed a greatly enlarged pit uitary gland with homogeneous pituitary tissue (Fig. 1). Elbow, knee and pelvis X-ray revealed that the bone age of the patient was 11 years classified based on the Greulich-Pyle system. The appearance was thought to be consistent with a pituitary macroadenoma and the patient was referred for an endocrinology opinion. Ophthalmological examinations, including visual fields test, were performed showing grossly intact and no proptosis. His thyroid gland was palpable and soft without any nodules. His chest X-ray was clear, and cardiac examination revealed a regular rhythm without murmurs. Neurologically, his reflexes were not delayed, and there was no tremor. His skin was cool and dry. He was prepubertal. His height was on the less than 3rd centile and weight on the 50th centile. Results of serial endocrinological parameters are shown in Table 1. He was diagnosed with normocytic anemia (hemoglobin 103 g/L), and hypercholesterolemia (8.42 mmol/L). Combined pituitary function test was also performed (Table 2). His TSH level was higher than 50 mIU/mL and free T4 was extremely low, at 0.05 ng/dL. Random growth hormone (GH) was less than 0.01 and insulin-like growth factor(IGF)-1 level was 12.66 nmol/L (normal 26.46~126.67). Goiter was noted and his anti-thyroid peroxidase (TPO) antibody level was elevated (> 3,000 U/mL, normal < 60 U/mL). Ultrasound scanning of the thyroid revealed a normally placed gland with increased vascularity. Diagnoses of severe Hashimoto's thyroiditis with secondary pituitary hyperplasia and growth hormone deficiency were finally made. The patient began oral levothyroxine (100 µg a day) and growth hormone replacement (3 mg, every each 5 days, Somatropin subcutaneous injection). The patient became clinically and biochemically euthyroid and his IGF-1 level was in normal range (46.78 nmol/L) (Table 1). Repeated MRI a fter 3 months of treatment showed dramatic reduction in the size of the pituitary gland to almost normal size (Fig. 1). His height initially increased to the level of 10th centile, then continued to increase along the 50th centile. In addition, he had signs of early pubertal development.
The chief complaint of the patient in this case was growth retardation. The discrepancy between bone age and chronologic age was 28 months. Based on further studies, he was diagnosed with primary hypothyroidism and growth hormone deficiency. Initially the pituitary mass was thought to be a macroadenoma and was considered for surgery is efficacious because of its location and size. On further evaluation, it was more likely that the mass might be caused by his long-standing untreated hypothyroidism. After six months of treatment with levothyroxine, he became clinically euthyroid and growth arrest was resolved. Follow up MRI showed the size of sellar mass was definitely decreased.
Sellar and suprasellar masses can be caused by several factors. However, it is clinically not easy to discriminate from many causes of sellar mass in children. There were several reasons we could not easily distinguish secondary pituitary hyperplasia from pituitary adenoma in this case. First, there is no specific diagnostic tool. MRI and CT have proven to have a low specificity in diagnosing pituitary hyperplasia and distinguishing it from pituitary adenomas[6]. Second, baseline concentration of alpha subunit was elevated. And thyrotropin-releasing hormone (TRH) stimulation test showed typically blunted response. National Institutes of Health study reported that baseline concentration of alpha subunit in the case series of 25 patients with TSH secreting tumors was generally elevated (range: 0.9~135 µg/L). Serum alpha subunit in patients with pituitary hyperplasia never reached the level observed in subjects having primary pituitary tumors[7]. Another series showed that only four of nine patients with TSH-producing tumors had normal TSH responses to the TRH stimulation test[8]. Third, the mass size was larger than any other previous reports. Earlier reports indicated that physiological hypertrophy of the pituitary gland may be one of the differential diagnoses in this kind of patient. One study reported that relatively large mean height of the pituitary gland (8.2 mm) in an adolescent female patient on CT scans represented pubertal hypertrophy[9]. Furthermore Elster et al. reported definite evidence of physiological hypertrophy of the pituitary gland could be detected by MRI and the average height of the pituitary gland of a teenage boy was 8~10 mm[10]. In our patient, the height was 18.1 mm, which is much larger than the average height when there is physiological hypertrophy of the pituitary gland and also larger than any other secondary pituitary hyperplasia previously reported[11].
Despite these reasons, we preferred pituitary hyperplasia rather than pituitary tumor. First, this patient had already diagnosed with hypothyroidism. Second, he had no specific neurological presentation. Third, alpha subunit/TSH ratio of the present patient was less than 1. In fact, the clinical diagnosis of hypothyroidism is often delayed and the increased availability of MRI may lead to more cases presenting with pituitary findings. Interpretation of the brain imaging as a primary pituitary tumor without any evidence of endocrinological laboratory parameters can lead to unnecessary surgery with potential catastrophic results[5]. Therefore, serum TSH and prolactin levels should be appropriately measured in all patients found to have a pituitary mass.
Unlike adults, children rarely have neurological presentations secondary to sellar expansion[12]. In prepubescent children, at the age ranging 8 to 14, pituitary hyperplasia was present in both boys as well as girls. Most children presented with signs of growth arrest. TSH levels were elevated with low free T4 concentrations along with enlargement of the sella turcica. From review of the literature, it appears that similar high doses of thyroid hormone replacement therapy were required for treatment. However, longer periods of treatment were needed for pituitary regression. Fortunately, congenital hypothyroidism and pituitary hyperplasia are rarely found in children in developed countries due to increased neonatal screening.
It is also interesting that this patient had hyperprolactinemia. Hyperprolactinemia is a common feature in patients with hypothyroidism[13]. The hyperprolactinemia seems to be of limited clinical significance in adults[14] and often, but not always, resolves with thyroid hormone replacement[15]. The pathophysiology of the increased serum prolactin level is not clear, however it may be attributed to a mass effect, the 'stalk effect'[16], or elevated TRH levels[17]. Pure prolactinomas can also present with mass effects and MRI findings rather than endocrine manifestations. These pituitary adenomas tend to respond well to medical treatment and can usually be diagnosed on the basis of biochemical results. Cases of superfluous surgical interventions without full endocrinological evaluation have been previously described[4,5,19].
Another important finding is that, on admission, the level of IGF-1 and GH were decreased. However, 2 month after levothyroxine and growth hormone replacement, IGF-1 level was normalized. Furthermore, his height increased as the level of 50th centile. Even though we did not check up the level of GH again, another report demonstrated that the level of GH was normalized after thyroid hormone replacement. It might suggest that GH deficiency was caused by hypothyroidism and it was just temporary[18]. For further evaluation, we plan to perform combined pituitary function test again after discontinue GH.
Our patient had many findings similar to those previously reported. However, the concentration of alpha subunit, alpha subunit/TSH ratio was much higher than normal range and mass size was very huge comparing to other patients. As a consequence, physician who first met him could not easily differentiate pituitary hyperplasia from pituitary tumor.
The pituitary hyperplasia associated with hypothyroidism responds well to thyroid hormone replacement therapy. Normalization of the serum TSH with thyroxine replacement in hypothyroidism associated pituitary hyperplasia predicts regression of the mass, excluding the presence of autonomous TSH secretion. Confirmation of resolution of the mass should be sought by monitoring visual fields and performing MRI after treatment. Thus, careful history taking, and reexamination of thyroid function in patients with sellar and suprasellar masses revealed by MRI may prevent unnecessary operations.
Figures and Tables
Fig. 1
Brain magnetic resonance imaging (MRI) before treatment and after treatment. Interval change of brain MRI at presentation (A, B) and after 3 months of thyroxine treatment (C, D) (Enlarged pituitary glands with homogeneous pituitary tissue mimicking a pituitary macroadenoma).
References
1. Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am. 1999. 28:81–117.
2. Atchison JA, Lee PA, Albright AL. Reversible suprasellar pituitary mass secondary to hypothyroidism. JAMA. 1989. 262:3175–3177.
3. Khawaja NM, Taher BM, Barham ME, Naser AA, Hadidy AM, Ahmad AT, Hamamy HA, Yaghi NA, Ajlouni KM. Pituitary enlargement in patients with primary hypothyroidism. Endocr Pract. 2006. 12:29–34.
4. Khalil A, Kovacs K, Sima AA, Burrow GN, Horvath E. Pituitary thyrotroph hyperplasia mimicking prolactin-secreting adenoma. J Endocrinol Invest. 1984. 7:399–404.
5. Torpiano J, Vanderpump M, Stanhope R. The management of sellar masses: not all pituitary tumours require surgery for diagnosis and/or therapy. J Pediatr Endocrinol Metab. 2004. 17:663–664.
6. Sarlis NJ, Brucker-Davis F, Doppman JL, Skarulis MC. MRI-demonstrable regression of a pituitary mass in a case of primary hypothyroidism after a week of acute thyroid hormone therapy. J Clin Endocrinol Metab. 1997. 82:808–811.
7. Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab. 1999. 84:476–486.
8. Gesundheit N, Petrick PA, Nissim M, Dahlberg PA, Doppman JL, Emerson CH, Braverman LE, Oldfield EH, Weintraub BD. Thyrotropin-secreting pituitary adenomas: clinical and biochemical heterogeneity: case reports and follow-up of nine patients. Ann Intern Med. 1989. 111:827–835.
9. Peyster RG, Hoover ED, Viscarello RR, Moshang T, Haskin ME. CT appearance of the adolescent and preadolescent pituitary gland. Am J Neuroradiol. 1983. 4:411–414.
10. Elster AD, Chen MY, Williams DW 3rd, Key LL. Pituitary gland: MR imaging of physiologic hypertrophy in adolescence. Radiology. 1990. 174:681–685.
11. Joshi AS, Woolf PD. Pituitary hyperplasia secondary to primary hypothyroidism: a case report and review of the literature. Pituitary. 2005. 8:99–103.
12. Desai MP, Mehta RU, Choksi CS, Colaco MP. Pituitary enlargement on magnetic resonance imaging in congenital hypothyroidism. Arch Pediatr Adolesc Med. 1996. 150:623–628.
13. Grubb MR, Chakeres D, Malarkey WB. Patients with primary hypothyroidism presenting as prolactinomas. Am J Med. 1987. 83:765–769.
14. Raber W, Gessl A, Nowotny P, Vierhapper H. Hyperprolactinaemia in hypothyroidism: clinical significance and impact of TSH normalization. Clin Endocrinol (Oxf). 2003. 58:185–191.
15. Ozbey N, Sariyildiz E, Yilmaz L, Orhan Y, Sencer E, Molvalilar S. Primary hypothyroidism with hyperprolactinaemia and pituitary enlargement mimicking a pituitary macroadenoma. Int J Clin Pract. 1997. 51:409–411.
16. Nomikos P, Buchfelder M, Fahlbusch R. Current management of prolactinomas. J Neurooncol. 2001. 54:139–150.
17. Hung W, Fitz CR, Lee ED. Pituitary enlargement due to lingual thyroid gland and primary hypothyroidism. Pediatr Neurol. 1990. 6:60–62.
18. Kim DH, Chung SC, Kim HS. Two cases of pituitary hyperplasia secondary to primary hypothyroidism mimicking pituitary tumor. J Korean Soc Pediatr Endocrinol. 1997. 2:241–247.
19. Samaan NA, Osborne BM, Mackay B, Leavens ME, Dulleo TM, Halmi NS. Endocrine and morphologic studies of pituitary adenomas secondary to primary hypothyroidism. J Clin Endocrinol Metab. 1977. 45:903–911.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download