Figures and Tables
References
1. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000. 404:661–671.
2. National Task Force on the Prevention and Treatment of Obesity: Overweight, obesity, and health risk. Arch Intern Med. 2000. 160:898–904.
3. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997. 20:537–544.
4. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group.Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001. 344:1343–1350.
5. Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP-NIDDM Trial: an international study on the efficacy of an alpha-glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: rationale, design, and preliminary screening data. Study to Prevent Non-Insulin-Dependent Diabetes Mellitus. Diabetes Care. 1998. 21:1720–1725.
6. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002. 25:2165–2171.
7. Plodkowski RA, St Jeor ST. Medical nutrition therapy for the treatment of obesity. Endocrinol Metab Clin North Am. 2003. 32:935–969.
8. National Task Force on the Prevention and Treatment of Obesity: Long-term pharmacotherapy in the management of obesity. JAMA. 1996. 276:1907–1915.
9. Yanovski SJ, Yanovski JA. Obesity. N Engl J Med. 2002. 346:591–602.
10. WHO Western Pacific Region. International Obesity Task Force: The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. International Association for the Study of Obesity. 2000. Sydney, Australia: Health Communications Australia;15–21.
11. Hensrud DD. Pharmacotherapy for obesity. Med Clin North Am. 2000. 84:463–476.
12. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007. 369:71–77.
13. Bray GA. Use and abuse of appetite-suppressant drugs in the treatment of obesity. Ann Intern Med. 1993. 119:707–713.
14. Weintraub M, Hasday JD, Mushlin AI, Lockwood DH. A double-blind clinical trial in weight control: use of fenfluramine and phentermine alone and in combination. Arch Intern Med. 1984. 144:1143–1148.
15. Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995. 57:411–441.
16. Stock MJ. Sibutramine: a review of the pharmacology of a novel anti-obesity agent. Int J Obes. 1997. 21:Suppl 1. S25–S29.
17. Ryan DH. Use of sibutramine and other noradrenergic and serotonergic drugs in the management of obesity. Endocrine. 2000. 13:193–199.
18. Hansen DL, Toubro S, Stock MJ, Macdonald IA, Astrup A. The effect of sibutramine on energy expenditure and appetite during chronic treatment without dietary restriction. Int J Obes Relat Metab Disord. 1999. 23:1016–1024.
19. Walsh KM, Leen E, Lean ME. The effect of sibutramine on resting energy expenditure and adrenaline-induced thermogenesis in obese females. Int J Obes Relat Metab Disord. 1999. 23:1009–1015.
20. Fujioka K, Seaton TB, Rowe E, Jelinek CA, Raskin P, Lebovitz HE, Weinstein SP. Sibutramine/Diabetes Clinical Study Group: Weight loss with sibutramine improves glycaemic control and other metabolic parameters in obese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2000. 2:175–187.
21. Finer N, Bloom SR, Frost GS, Banks LM, Griffiths J. Sibutramine is effective for weight loss and diabetic control in obesity with type 2 diabetes: a randomised, double-blind, placebo-controlled study. Diabetes Obes Metab. 2000. 2:105–112.
22. McNulty SJ, Ur E, Williams G. Multicenter Sibutramine Study Group: A randomized trial of sibutramine in the management of obese type 2 diabetic patients treated with metformin. Diabetes Care. 2003. 26:125–131.
23. Sharma AM. Sibutramine in overweight/obese hypertensive patients. Int J Obes Relat Metab Disord. 2001. 24:Suppl 4. S20–S23.
24. Hazenberg BP. Randomized, double-blind, placebo-controlled, multicenter study of sibutramine in obese hypertensive patients. Cardiology. 2000. 94:152–158.
25. Fanghänel G, Cortinas L, Sánchez-Reyes L, Gómez-Santos R, Campos-Franco E, Berber A. Safety and efficacy of sibutramine in overweight Hispanic patients with hypertension. Adv Ther. 2003. 20:101–113.
26. Jordan J, Scholze J, Matiba B, Wirth A, Hauner H, Sharma AM. Influence of sibutramine on blood pressure: evidence from placebo-controlled trials. Int J Obes Relat Metab Disord. 2005. 29:509–516.
27. Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000. 20:270–279.
28. Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, Heimburger DC, Lucas CP, Robbins DC, Chung J, Heymsfield SB. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999. 281:235–242.
29. Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, Weiss SR, Crockett SE, Kaplan RA, Comstock J, Lucas CP, Lodewick PA, Canovatchel W, Chung J, Hauptman J. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care. 1998. 21:1288–1294.
30. Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008. 31:53–65.
31. Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol Rev Camb Philos Soc. 2004. 79:187–205.
32. De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004. 141:765–774.
33. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation, Nat Rev Dru. Discov. 2004. 3:771–784.
34. Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005. 8:585–589.
35. Osei-Hyiaman D, De Petrillo M, Pacher P, Piu J, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2006. 115:1298–1305.
36. Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002. 22:9612–9617.
37. Onaivi ES, Leonard CM, Ishiguro H, Zhang PW, Lin Z, Akinshola BE, Uhl GR. Endocannabinoids and cannabinoid receptor genetics. Prog Neurobiol. 2002. 66:307–344.
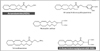
38. Boyd ST, Fremming BA. Rimonabant: a selective CB1 antagonist. Ann Pharmacother. 2005. 39:684–690.
39. Gary-Bobo M, Elachouri G, Scatton G, Le Fur G, Oury-Donat F, Bensaid M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol. 2006. 69:471–478.
40. Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohórquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN 3rd, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depré M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008. 7:68–78.
41. Addy C, Li S, Agrawal N, Stone J, Majumdar A, Zhong L, Li H, Yuan J, Maes A, Rothenberg P, Cote J, Rosko K, Cummings C, Warrington S, Boyce M, Gottesdiener K, Stoch A, Wagner J. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, for the treatment of obesity: results from a double-blind, placebo-controlled, single oral dose study in healthy volunteers. J Clin Pharmacol. 2008. 48:418–427.
42. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S; RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005. 365:1389–1397.
43. Despres JP, Golay A, Sjöström L. Rimonabant in Obesity-Lipids Study Group: Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005. 353:2121–2134.
44. Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J; RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006. 295:761–775.
45. Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF; RIO-Diabetes Study Group. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomized controlled study. Lancet. 2006. 368:1660–1672.
46. Oh I, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006. 443:709–712.
47. Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007. 450:106–109.
48. Sloth B, Davidsen L, Holst JJ, Flint A, Astrup A. Effect of subcutaneous injections of PYY1-36 and PYY3-36 on appetite, ad libitum energy intake, and plasma free fatty acid concentration in obese males. Am J Physiol Endocrinol Metab. 2007. 293:E604–E609.
49. Gantz I, Erondu N, Mallick M, Musser B, Krishna R, Tanaka WK, Snyder K, Stevens C, Stroh MA, Zhu H, Wagner JA, MacNeil DJ, Heymsfield SB, Amatruda JM. Efficacy and safety of intranasal peptide YY3-36 for weight reduction in obese adults. J Clin Endocrinol Metab. 2007. 92:1754–1757.
50. Erondu N, Addy C, Lu K, Mallick M, Musser B, Gantz I, Proietto J, Astrup A, Toubro S, Rissannen AM, Tonstad S, Haynes WG, Gottesdiener KM, Kaufman KD, Amatruda JM, Heymsfield SB. NPY5R antagonism does not augment the weight loss efficacy of orlistat or sibutramine. Obesity (Silver Spring). 2007. 15:2027–2042.
51. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999. 341:879–884.
52. Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999. 282:1568–1575.
53. Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000. 85:4003–4009.
54. Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008. 93:2027–2034.
55. Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997. 337:581–588.
56. Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000. 343:1826–1832.
57. Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000. 343:1833–1838.
58. Sharma AM. Sibutramine in overweight/obese hypertensive patients. Int J Obes Relat Metab Disord. 2001. Dec. 25:Suppl 4. S20–S23.
59. James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rössner S, Saris WH, Van Gaal LF. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000. 356:2119–2125.
60. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004. 27:155–161.
61. Kelley DE, Kuller LH, McKolanis TM, Harper P, Mancino J, Kalhan S. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care. 2004. 27:33–40.
62. Torp-Pedersen C, Caterson I, Coutinho W, Finer N, Van Gaal L, Maggioni A, Sharma A, Brisco W, Deaton R, Shepherd G, James P. SCOUT Investigators: Cardiovascular responses to weight management and sibutramine in high-risk subjects: an analysis from the SCOUT trial. Eur Heart J. 2007. 28:2915–2923.
63. Sharma AM, Caterson ID, Coutinho W, Finer N, Van Gaal L, Maggioni AP, Torp-Pedersen C, Bacher HP, Shepherd GM, James WP. SCOUT Investigators: Blood pressure changes associated with sibutramine and weight management - an analysis from the 6-week
lead-in period of the sibutramine cardiovascular outcomes trial (SCOUT). Diabetes Obes Metab. 2008. Jul. 29. [Epub ahead of print].
64. Comprehensive Rimonabant Evaluation Study of Cardiovascular ENDpoints and Outcomes(CRESCENDO. ClinicalTrials.gov;Identifier: NCT00263042 (sanofi-aventis)
http://clinicaltrials.gov/ct/show/NCT00263042.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download