Figures and Tables
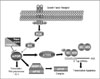
Fig. 3
Molecular target of rapamycin (mTOR) signaling pathway.
Adopted from Curr Opin Oncol 16: 564-575, 2004
References
1. Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001. 344:1038–1042.
2. Mizukami Y, Nonomura A, Hashimoto T, Kawahara E, Matsukawa S, Matsubara F. Immunohistochemical demonstration of ras p21 oncogene product in normal, benign, and malignant human thyroid tissues. Cancer. 1988. 61:873–880.
3. Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990. 4:1474–1479.
4. Lemoine NR, Mayall ES, Wyllie FS, Williams ED, Goyns M, Stringer B, Wynford-Thomas D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989. 4:159–164.
5. KlLoog Y, Cox AD. Ras inhibitor: potential for cancer therapeutics. Mol Med Today. 2000. 6:398–402.
6. Cunningham CC, Holmlund JT, Geary RS, Kwoh TJ, Dorr A, Johnston JF, Monia B, Nemunaitis J. A Phase I trial of H-ras antisense oligonucleotide ISIS 2503 administered as a continuous intravenous infusion in patients with advanced carcinoma. Cancer. 2001. 92:1265–1271.
7. Ringel MD, Greenberg M, Chen X, Hayre N, Suzuki K, Priebat D, Saji M, Burman KD. Cytotoxic activity of 2',2'-difluorodeoxycytidine (gemcitabine) in poorly differentiated thyroid carcinoma cells. Thyroid. 2000. 10:865–869.
8. Eigelberger MS, Wong MG, Duh QY, Clark OH. Phenylacetate enhances the antiproliferative effect of retinoic acid in follicular thyroid cancer. Surgery. 2001. 130:931–935.
9. Kebebew E, Wong MG, Siperstein AE, Duh QY, Clark OH. Phenylacetate inhibits growth and vascular endothelial growth factor secretion in human thyroid carcinoma cells and modulates their differentiated function. J Clin Endocrinol Metab. 1999. 84:2840–2847.
10. Hara M, Akasaka K, Akinaga S, Okabe M, Nakano H, Gomez R, Wood D, Uh M, Tamanoi F. Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc Natl Acad Sci USA. 1993. 90:2281–2285.
11. Yeung SC, Xu G, Pan J, Christgen M, Bamiagis A. Manumycin enhances the cytotoxic effect of paclitaxel on anaplastic thyroid carcinoma cells. Cancer Res. 2000. 60:650–656.
12. Xu G, Pan J, Martin C, Yeung SC. Angiogenesis inhibition in the in vivo antineoplastic effect of manumycin and paclitaxel against anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2001. 86:1769–1777.
13. Fagin J. How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol. 2004. 183:249–256.
14. Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003. 88:5399–5404.
15. Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R. B-RAF is a therapeutic target in melanoma. Oncogene. 2004. 23:6292–6298.
16. Braga-Basaria Milena, Ringel Matthew D. Beyond Radioiodine: A Review of Potential New Therapeutic Approaches for Thyroid Cancer. J Clin Endocrinol Metab. 2003. 88:1947–1960.
17. Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR, Saltiel AR. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999. 5:810–816.
18. Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, Hamid O, Varterasian M, Asbury P, Kaldjian EP, Gulyas S, Mitchell DY, Herrera R, Sebolt-Leopold JS, Meyer MB. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004. 22:4456–4462.
19. Santoro M, Melillo RM, Carlomagno F, Fusco A, Vecchio G. Molecular mechanisms of RET activation in human cancer. Annals of the New York Academy of Sciences. 2002. 963:116–121.
20. Kanamori A, Abe Y, Yajima Y, Manabe Y, Ito K. Epidermal growth factor receptors in plasma membranes of normal and diseased human thyroid glands. J Clin Endocrinol Metab. 1989. 68:899–903.
21. Akslen LA, Myking AO, Salvesen H, Varhaug JE. Prognostic impact of EGF-receptor in papillary thyroid carcinoma. Br J Cancer. 1993. 68:808–812.
22. Kremser R, Obrist P, Spizzo G, Erler H, Kendler D, Kemmler G, Mikuz G, Ensinger C. Her2/neu overexpression in differentiated thyroid carcinomas predicts metastatic disease. Virchows Arch. 2003. 442:322–328.
23. Ensinger C, Prommegger R, Kendler D, Gabriel M, Spizzo G, Mikuz G, Kremser R. Her2/neu expression in poorly-differentiated and anaplastic thyroid carcinomas. Anticancer Res. 2003. 23:2349–2353.
24. Holting T, Siperstein AE, Clark OH, Duh QY. Epidermal growth factor (EGF) and transforming growth factor alpha-stimulated invasion and growth of follicular thyroid cancer cells can be blocked by antagonism to the EGF receptor and tyrosine kinase in vitro. Eur J Endocrinol. 1995. 132:229–235.
25. Gabler B, Aicher T, Heiss P, Senekowitsch-Schmidtke R. Growth inhibition of human papillary thyroid carcinoma cells and multicellular spheroids by anti-EGF-receptor antibody. Anticancer Res. 1997. 17:3157–3159.
26. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–2139.
27. Schiff BA, McMurphy AB, Jasser SA, Younes MN, Doan D, Yigitbasi OG, Kim S, Zhou G, Mandal M, Bekele BN, Holsinger FC, Sherman SI, Yeung SC, El-Naggar AK, Myers JN. Epidermal growth factor receptor (EGFR) is overexpressed in anaplastic thyroid cancer, and the EGFR inhibitor gefitinib inhibits the growth of anaplastic thyroid cancer. Clin Cancer Res. 2004. 10:8594–8602.
28. Nobuhara Y, Hirakawa K. Efficacy of epidermal growth factor receptor-targeted molecular therapy in anaplastic thyroid cancer cell lines. Br J Cancer. 2005. 92:1110–1116.
29. Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for the Flk-1/KDR activation. J Biol Chem. 1998. 273:30336–30343.
30. Huang SM, Lee JC, Wu TJ, Chow NH. Clinical relevance of vascular endothelial growth factor for thyroid neoplasms. World J Surg. 2001. 25:302–306.
31. Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plenat F, Leclere J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a preoperative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001. 86:656–658.
32. Tuttle RM, Fleisher M, Francis GL, Robbins RJ. Serum vascular endothelial growth factor levels are elevated in metastatic differentiated thyroid cancer but not increased by short-term TSH stimulation. J Clin Endocrinol Metab. 2002. 87:1737–1742.
33. Borgstrom P, Hillan KJ, Sriramarao P, Ferrara N. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res. 1996. 56:4032–4039.
34. Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, Reimann JD, Vassel A. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003. 30:Suppl. 16. 117–124.
35. Bauer AJ, Terrell R, Doniparthi NK, Patel A, Tuttle RM, Saji M, Ringel MD, Francis GL. Vascular endothelial growth factor monoclonal antibody inhibits growth of anaplastic thyroid cancer xenografts in nude mice. Thyroid. 2002. 12:953–961.
36. Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999. 59:99–106.
37. Shaheen RM, Davis DW, Liu W, Zebrowski BK, Wilson MR, Bucana CD, McConkey DJ, McMahon G, Ellis LM. Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumor and endothelial cell apoptosis. Cancer Res. 1999. 59:5412–5416.
38. Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ESE, Curry B, Richmond GHP, Wadsworth PF, Bigley AL, Hennequin LF. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002. 62:4645–4655.
39. Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortura G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002. 62:7284–7290.
40. Zwingenberger K, Wnendt S. Immunomodulation by thalidomide: Systematic review of the literature and of unpublished observations. J Inflamm. 1995. 46:177–211.
41. D'Amato RJ, Loughnan MS, Flynn E. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994. 91:4082–4085.
42. Dimopoulos MA, Anagnostopoulos A, Weber D. Treatment of plasma cell dyscrasias with thalidomide and its derivatives. J Clin Oncol. 2003. 21:4444–4454.
43. Dziba JM, Marcinek R, Venkataraman G, Robinson JA, Ain KB. Combretastatin A4 phosphate has primary antineoplastic activity against human anaplastic thyroid carcinoma cell lines and xenograft tumors. Thyroid. 2002. 12:1063–1070.
44. Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, Rafie N, Overmoyer B, Makkar V, Stambler B, Taylor A, Waas J, Lewin JS, McCrae KR, Remick SC. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin A-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002. 62:3408–3416.
45. Panwalker A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004. 100:657–666.
46. Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001. 98:10314–10319.
47. Burman KD, Djuh YY, LaRocca RV, Nunes ME, D'Avis JC, Nicholson DE, Wartofsky L. c-Myc expression in the thyroid. I: Normal, adenomatous, and cancerous thyroid tissue. Horm Metab Res. 1987. 17:Suppl. 63–65.
48. Romano MI, Grattone M, Karner MP, Moiguer S, Tetelbaum F, Romano LA, Illescas E, Padin R, Cueva F, Burdman JA. Relationship between the level of c-myc mRNA and histologic aggressiveness in thyroid tumors. Horm Res. 1993. 39:161–165.
49. Erickson LA, Jin L, Wollan PC, Thompson GB, van Heerden J, Lloyd RV. Expression of p27kip1 and Ki-67 in benign and malignant thyroid tumors. Mod Pathol. 1998. 11:169–174.
50. Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD, Saji M. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001. 61:6105–6111.
51. Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997. 16:64–67.
52. Bruni P, Boccia A, Baldassarre G, Trapasso F, Santoro M, Chiappetta G, Fusco A, Viglietto G. PTEN expression is reduced in a subset of sporadic thyroid carcinomas: evidence that PTEN-growth suppressing activity in thyroid cancer cells mediated by p27kip1. Oncogene. 2000. 19:3146–3155.
53. Frisk T, Foukakis T, Dwight T, Lundberg J, Hoog A, Wallin G, Eng C, Zedenius J, Larsson C. Silencing of the PTEN tumor-suppressor gene in anaplastic thyroid cancer. Genes Chromosomes Cancer. 2002. 35:74–80.
54. Eng CP, Sehgal SN, Vezina C. Activity of rapamycin (AY-22, 989) against transplanted tumors. J Antibiot. 1984. 37:1231–1237.
55. Abekoff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG. Clinical Oncology. 2004. 3rd ed. Philadelphia: Elsevier;34–36. 104–109.
56. Mitsiades N, Poulaki V, Mastorakos G, Tseleni-Balafouta ST, Kotoula V, Koutras DA, Tsokos M. Fas ligand expression in thyroid carcinomas: a potential mechanism of immune evasion. J Clin Endocrinol Metab. 1999. 84:2924–2932.
57. Ahmad M, Shi Y. TRAIL-induced apoptosis of thyroid cancer cells: potential for therapeutic intervention. Oncogene. 2000. 19:3363–3371.
58. Mitsiades N, Poulaki V, Tseleni-Balafouta S, Koutras DA, Stamenkovic I. Thyroid carcinoma cells are resistant to FAS-mediated apoptosis but sensitive tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000. 60:4122–4129.
59. Campos L, Sabido O, Rouault JP, Guyotat D. Effects of BCL-2 antisense oligo-deoxynucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood. 1994. 84:595–600.
60. Fenaux P, Chevret S, Guerci A, Fegueux N, Dombret H, Thomas X, Sanz M, Link H, Maloisel F, Gardin C, Bordessoule D, Stoppa AM, Sadoun A, Muus P, Wandt H, Mineur P, Whittaker JA, Fey M, Daniel MT, Castaigne S, Degos L. European APL group. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia. Leukemia. 2000. 14:1371–1377.
61. Tallmann MS. Curative therapeutic approaches to APL. Ann Hematol. 2004. 83:Suppl. 1. S81–S82.
62. Simon D, Korber C, Krausch M, Segering J, Groth P, Gorges R, Grunwald F, Muller-Gartner HW, Schmutzler C, Kohrle J, Roher HD, Reiners C. Clinical impact of retinoids in redifferentiation therapy of advanced thyroid cancer: final results of a pilot study. Eur J Nucl Med Mol Imaging. 2002. 29:775–782.
63. van Herle AJ, Agatep UL, Padua HI DN, Totanes TL, Canlapan DV, van Herle HML, Juillard GJF. Effects of 13 cisretinoic acid on growth and differentiation of human follicular carcinoma cells (UCLA RO 82 W-1) in vitro. J Clin Endocrinol Metab. 1990. 71:755–763.
64. Schreck R, Schnieders F, Schmutzler C, Kohrle J. Retinoids stimulate type 1 iodothyronine 5'-deiodinase activity in human follicular thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1994. 79:791–798.
65. Bassi V, Vitale Nt, Feliciello A, De Riu S, Rossi G, Fenzi G. Retinoic acid induces intercellular adhesion molecule-1 hyperexpression in human thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1995. 80:1129–1135.
66. Short SC, Suovuori A, Cook G, Vivian G, Harmer C. A phase II study using retinoids as redifferentiation agents to increase iodine uptake in metastatic thyroid cancer. Clin Oncol (R Coll Radiol). 2004. 16:569–574.
67. Tycko B. Epigenetic gene silencing in cancer. J Clin Invest. 2000. 105:401–407.
68. Venkataraman GM, Yatin M, Marcinek R, Ain KB. Restoration of iodide uptake in dedifferentiated thyroid carcinoma: Relationship to human Na+/I- symporter gene methylation status. J Clin Endocrinol Metab. 1999. 84:2449–2457.
69. Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, Bates S, Fojo T. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR 901228), increase expression of the Na+/I- symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. 2001. 86:3430–3435.
70. Specht MC, Tucker ON, Hocever M, Gonzalez D, Teng L, Fahey TJ III. Cyclo-oxygenase-2 expression in thyroid nodules. J Clin Endocrinol Metab. 2002. 87:358–363.
71. Masferrer JL, Leahy KM, Koki AT. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000. 60:1306–1311.
72. Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997. 99:2254–2259.
73. Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999. 59:2615–2622.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download