Abstract
Background
Pheochromocytomas and paragangliomas (PPGLs) are rare endocrine tumors originating from chromaffin cells. PPGLs are associated with a high mortality rate and several complications. To date, no epidemiological studies have been conducted on PPGLs in Asia. This study aimed to investigate the epidemiology and prognosis of PPGLs in Korea using nationwide data.
Methods
Using the National Health Insurance Service Database, subjects with a principal diagnosis of PPGLs on two or more occasions between 2003 and 2014 who satisfied the operational definition of PPGLs were included. Incidence, prevalence, complications, metastasis, and mortality were investigated.
Results
In total, 1048 subjects with a mean age of 47.6±16.1 years were included. There was no sex preponderance. The overall prevalence of PPGLs was 2.13 per 100,000 persons, and the overall age-standardized incidence rate was 0.18 per 100,000 person-years. Malignant PPGLs accounted for 17.7% (185 of 1,048) of cases, and 94 subjects exhibited metastasis at the time of diagnosis. Among initially non-metastatic PPGLs, 9.5% (nine of 954) eventually metastasized after a mean duration of 78.1±41.4 months. The 5-year survival rates for non-metastatic and metastatic PPGLs at diagnosis were 97% and 84%, respectively. Multivariable Cox regression models adjusted for covariates showed that metastatic PPGLs were associated with a 2.40-fold higher risk of mortality than non-metastatic PPGLs (95% confidence interval, 1.38 to 4.17; P=0.002).
Pheochromocytomas and paragangliomas (PPGLs) are rare endocrine tumors that originate from chromaffin cells of the sympathetic and parasympathetic nervous systems. Pheochromocytomas (PCCs) most often arise from the adrenal medulla, while paragangliomas (PGLs) tend to arise from the sympathetic or parasympathetic ganglia outside of the adrenal medulla, such as those in the retroperitoneum, pelvis, thorax, head, and neck [1]. While PCCs are commonly functional, in which case they produce one or more catecholamines, PGLs derived from the parasympathetic ganglia are often non-functional. Typical PPGL symptoms that occur due to excess catecholamines include headaches, palpitations, paroxysmal hypertension, and sweating. In addition to these symptoms, cardiovascular and metabolic-related morbidity and mortality also increase due to catecholamine excess [2345]. Furthermore, some PPGLs have metastatic potential. Metastatic PPGLs are defined by the presence of chromaffin cell-derived tumors in non-chromaffin organs at diagnosis or during follow-up. In the past, metastatic PPGLs have accounted for 10% to 20% of all PPGLs [67].
Among patients with hypertension, the prevalence of PPGLs varies between 0.2% and 0.6%. Approximately 5% to 7% of patients with adrenal incidentalomas are diagnosed with PCCs [89]. In autopsy cases, PCC was recognized in one tumor among 2031 autopsies (0.05%) [10]. However, epidemiological studies are rarely conducted on the level of a national population. A national pathology study conducted in the Netherlands between 1995 and 2015 identified 1,493 patients with PCC or sympathetic PGL, corresponding to an age-standardized incidence rate of 0.57 per 100,000 person-years [11]. The overall incidence of PPGLs has been reported to be gradually increasing over time [11].
Moreover, to date, no epidemiological studies on PPGLs have been conducted in Korea. Therefore, the aim of this study was to investigate and identify the epidemiology of PPGLs in Korea and to explore related complications and prognoses using nationwide data based on the National Health Insurance Service (NHIS).
The Korean National Health Insurance Program, which is operated by the Ministry for Health, Welfare and Family Affairs, covers nearly the entire Korean population (specifically, around 97%) [12]. All medical institutions submit healthcare utilization-related data to ensure reimbursements, and these data are stored in the NHIS database. Therefore, the NHIS database contains all forms of healthcare data, including data on demographic characteristics, diagnoses, comorbidities (as defined by the International Classification of Diseases, 10th revision [ICD-10]), prescriptions, diagnostic or surgical procedures, and the medical costs of claims. The data used for the present study comprised all NHIS claim records between 2002 and 2014.
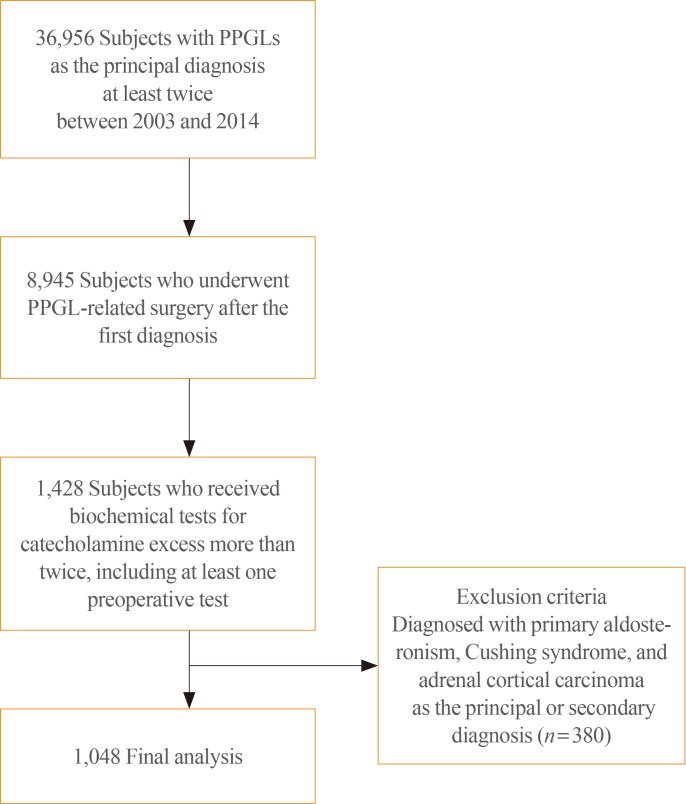
First, a 1-year window (from January 1, 2002 to December 31, 2002) was set. Subjects with their first claim record in 2002 (n=71), which might have included those diagnosed before 2002, were excluded from this study. Subjects with PPGLs who satisfied the following criteria from January 1, 2003 to December 31, 2014 were included: (1) having the following ICD-10 diagnostic codes as the principal diagnosis on two or more—occasions: D350 (benign neoplasm of adrenal gland), D441 (neoplasm of uncertain behavior, adrenal), I1522 (hypertension due to PCC), C741 (malignant neoplasm, adrenal medulla), or C749 (malignant neoplasm, adrenal nonspecified) for adrenal tumors; D356 (benign neoplasm, aortic body, and PGL), D446 (neoplasm of uncertain behavior, carotid), D447 (neoplasm of uncertain behavior, aortic body, and PGL), D487 (neoplasm of uncertain behavior, unspecific sites), or C755 (malignant neoplasm, aortic body, and PGL) for PGLs. Subjects with one of the above-mentioned diagnoses as the primary diagnosis on two or more occasions were included (n=36,956). The first date of PPGL registration in the NHIS database was assumed to be the date of diagnosis. (2) Identified subjects were further limited to those who were undergoing the following types of surgery after the first date of PPGL registration (n=8,945): P4571 (adrenalectomy, unilateral), P4572 (adrenalectomy, bilateral), P4581 (carotid body tumor resection, unilateral), P4582 (carotid body tumor resection, bilateral), Q2501 (retroperitoneal tumor resection, unilateral), Q2502 (retroperitoneal tumor resection, bilateral), R3512 (transurethral resection of bladder tumor), O1591 (mediastinal tumor resection, unilateral), or O1592 (mediastinal tumor resection, bilateral). (3) Among these, subjects who underwent biochemical tests for catecholamine excess, such as tests for vanillylmandelic acid (C3211, C3212, C3213), epinephrine (C3231), norepinephrine (C3232), dopamine (C3233), normetanephrine (C3234), metanephrine (C3235), or total catecholamines (C3239) at least twice, including at least one preoperative biochemical test, were included (n=1,428). (4) Of the identified cases, patients with primary aldosteronism (E260, I1520, I1521), Cushing syndrome (E240, E248, E249), or adrenal cortical carcinoma (C740) as the principal or secondary diagnosis were excluded (n=380). A total of 1,048 subjects who satisfied all inclusion and exclusion criteria were included (Fig. 1). Among them, metastatic PPGLs were defined as being present in cases with the diagnostic codes of C741, C749, and C755 at least once. Subjects with the diagnostic codes of C741, C749, or C755 at diagnosis were considered to have metastasis at diagnosis. Subjects who had no metastasis at the time of first diagnosis, but in whom metastasis was detected during the follow-up period, were classified as having experienced metastasis during follow-up. Subjects with parasympathetic head and neck PGLs were not included, since these tumors are nonfunctional and may not show a response on biochemical tests.
Comorbidities were defined if the following diagnostic codes were present as at least two principal or secondary diagnoses, regardless of the date of diagnosis: cerebrovascular disease (I60, I61, I62, I63, I64, I67), cardiovascular disease (I11, I20, I21, I25.1, I50), aortic disease (I71), diabetes mellitus (E10, E11, E12, E13, E14), osteoporosis (M80, M81), and fractures (M48.4, S42.2, S52.0, S52.1, S52.2, S72.0, S72.1, S72.2, S32.0, S32.1, S32.2, S32.5, S22.0).
The subjects' baseline demographic and clinical characteristics were summarized as mean±standard deviation for interval variables and as numbers (%) for categorical variables. Age-specific incidence rates of PPGLs were calculated by dividing the number of cases in the specific age group by the corresponding age-specific national population. Mortality data obtained from the National Statistical Office were used to determine the survival status of study subjects after PPGL diagnosis. Cox proportional hazard regression models were applied to compare the mortality risk between metastatic and non-metastatic PPGLs. Multivariable Cox proportional hazard regression models were adjusted for age, sex, cerebrovascular disease, cardiovascular disease, diabetes mellitus, and fractures. The proportionality of hazards assumption between metastatic and non-metastatic PPGL groups was examined using a logarithm plot of cumulative hazards, and the assumption was found to be met. Hazard ratios (HRs) and 95% confidence intervals (CI) were calculated. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and STATA version 15.0 (Stata Corp., College Station, TX, USA). All P values provided were two-sided, and P values <0.05 were considered to indicate statistical significance.
Personal information in the Health Insurance Review and Assessment Service database was anonymously encrypted. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 1803-033-926). Informed consent was waived by the IRB.
Between 2003 and 2014, there were a total of 1,048 subjects with PPGLs, with a mean age of 47.6±16.1 years. The peak age of onset varied between 40 and 59 years. There was no sex preponderance (men, n=517 [49.5%]; women, n=528 [50.5%]) (Fig. 2). The overall prevalence of PPGLs was 2.13 per 100,000 persons, while the overall age-standardized incidence rate was 0.18 per 100,000 person-years. The number of subjects with PPGLs was lowest in 2004 (n=54) and highest in 2011 (n=123), and the crude incidence rate showed a similar tendency (Fig. 3).
PPGLs were accompanied by a variety of comorbidities. When all complications were included regardless of the date of diagnosis, the prevalence rates of cerebrovascular disease, cardiovascular disease, aortic disease, diabetes mellitus, osteoporosis, and fractures were 15.5%, 39.1%, 0.7%, 56.8%, 25.3%, and 6.2%, respectively (Table 1). The overall rate of cardiovascular-related morbidity was approximately 55%.
Further analysis was performed with regard to patients' prognosis. At the time of diagnosis, 9.0% (94/1,048) of PPGLs had already metastasized to other organs. Among initially benign PPGLs (n=954), 9.5% (n=91) eventually metastasized to other organs. The mean duration to progression into metastatic lesions was 78.1±41.4 months (Fig. 4). The overall rate of PPGLs showing malignancy at initial diagnosis or during the follow-up period was 17.7% (185 of 1,048).
A total of 61 deaths among 1,048 subjects were reported during a median follow-up duration of 78.8 months (range, 7.9 to 168). Among the 94 subjects who had metastatic PPGLs at diagnosis, 15 died, whereas among the 954 subjects with non-metastatic PPGLs at diagnosis, 46 died. Among the 91 subjects who had initially benign PPGLs, but experienced metastasis during the follow-up period, nine subjects died. The 5-year survival rates for non-metastatic and metastatic PPGLs at diagnosis were 97% and 84%, respectively. The HR for mortality was 2.86 (95% CI, 1.59 to 5.15) for metastatic PPGLs at diagnosis when compared with non-metastatic PPGLs at diagnosis (n=954), with the latter group including 91 patients who experienced metastasis during follow-up (Fig. 5). The HR for mortality was not significantly different between subjects with non-metastatic PPGLs (n=863) and those who had initially benign PPGLs, but experienced metastasis during the follow-up period (n=91) (data not shown, HR, 1.25; 95% CI, 0.59 to 2.65). Table 2 shows that the HR for subjects with metastatic PPGLs at diagnosis or during the follow-up period (n=185) was 2.04 (95% CI, 1.20 to 3.48) when compared with those with non-metastatic PPGLs (n=863). The mean survival time of subjects with metastatic PPGLs at diagnosis was 97.4±51.0 months. In multivariable Cox regression models adjusted for age, sex, cerebrovascular disease, cardiovascular disease, diabetes mellitus, and fractures, metastatic PPGLs at diagnosis or during the follow-up period were associated with a 2.40-fold higher risk of mortality than non-metastatic PPGLs (95% CI, 1.38 to 4.17; P=0.002) (Table 2). Male subjects and those with cerebrovascular disease had particularly high risk of mortality.
This was the first nationwide population-based epidemiological study of PPGLs to be conducted in Asia, using NHIS data from Korea. In this study, PPGLs were found to have a prevalence of 2.13 per 100,000 persons and an age-standardized incidence of 0.18 per 100,000 person-years. Past studies have reported the prevalence of PPGLs to range from 0.2% to 0.6% in hypertensive patients [13141516]. The incidence rate of PCCs was estimated to be 0.8 per 100,000 person-years (11 cases for 30 years in 45,800 people) in Rochester, Minnesota [17]. However, only a few epidemiological studies have been conducted using a nationwide registry. A recent nationwide pathology registry study conducted in the Netherlands identified a total of 1,493 patients with either PCC or sympathetic PGL between 1995 and 2015 [11]. The researchers reported overall age-standardized incidence rates of 0.37 and 0.57 per 100,000 person-years in 1995–1999 and 2011–2015, respectively [11]. These incidence rates were higher than that reported in the present study (0.18 per 100,000 person-years). The Dutch group reported an increased incidence of PPGLs over the past two decades, in contrast with the present study, which did not demonstrate any increasing trend with time. This difference is most likely due to the shorter study period in the present study than in the Dutch study. The mean age at diagnosis was 47.6 years in Korean PPGL patients, which is similar to or lower than that in previous studies [1118]. Similar to the present study, some studies reported no significant difference in prevalence between the sexes, while findings from another study demonstrated a slight female preponderance (55% vs. 45%) [1118].
Cardiovascular diseases such as arrhythmia, myocardial ischemia, heart failure, stress-induced cardiomyopathy, and stroke are major complications related to excessive circulating catecholamines [4]. In the present study, the approximate rate of cardiovascular and cerebrovascular disease was 55%, which is higher than the rate reported in a previous study [5]. Hyperglycemia and diabetes mellitus, observed in around 23% to 50% of patients, are some of the potential complications of catecholamine excess [1920]. Similar rates of diabetes mellitus (52.9%) were found in our PPGL subjects. Successful tumor resection resolved diabetes mellitus in 78.6% of patients [20]. Hyperglycemia in PPGL patients results from inhibited insulin secretion, stimulated glucagon secretion, and increased peripheral insulin resistance [19]. The present study revealed osteoporosis and fracture prevalence rates of 25.3% and 6.2%, respectively. With regards to bone health, trabecular bone loss and elevated bone resorption markers have been reported [21]. The apparent mechanism was sympathetic overactivation, which resulted in an uncoupling between excessive bone resorption and inadequate bone formation [22].
The present study demonstrated a high incidence of metastatic PPGLs in all study subjects (17.8%), which is similar to the rates reported in previous studies (8% to 20%) [672324]. However, a single-center study in Korea reported that among 223 PPGL patients, 12 (5.3%) presented with metastasis at diagnosis, whereas 17 (7.6%) developed metastasis during follow-up [6]. In another Korean study, the prevalence of metastatic PPGLs was 11.0% (n=33) among 299 PPGL patients [24]. The difference between these studies and the present study is most likely due to the different characteristics of the subjects. In the present study, due to the use of claims data, subjects were identified only based on diagnostic codes without pathological confirmation. Thus, pheochromocytomatosis—tumor spillage after adrenalectomy of benign PCCs—may have contributed to the high rate of metastasis [2526]. Nevertheless, subjects of the present study could not be lost to follow-up since the whole national population was included. This study also reported that 10.5% of subjects with non-metastatic PPGLs at the time of initial diagnosis developed metastatic lesions within a median duration of 6 years. Similarly, Hamidi et al. [23] also demonstrated the development of metastases within a median duration of 5.5 years (range, 0.3 to 53.4) from initial diagnosis [23]. This finding emphasized the importance of long-term follow-up, even in patients who initially present with non-metastatic PPGLs.
Previous reports on the 5-year survival rates for metastatic PPGLs have been widely inconsistent, ranging from 12% to 84% [272829]. A recent study reported a 5-year survival rate of 85.4% in 272 subjects with metastatic PPGLs [23]. In the present study, the 5-year survival rate for metastatic PPGLs was 84%. Individualized treatment for malignant PPGLs is therefore required, and the heterogeneity of metastatic PPGLs must be emphasized.
The present study had several strengths. First, it explored nationwide data representative of the Korean population and demonstrated the overall prevalence and incidence of PPGLs in Korea. Additionally, enrolled subjects could not be lost to follow-up, since health insurance is mandatory in the Korean population. The use of complete and long-term follow-up data helped in the evaluation of real-world mortality risk.
However, several inevitable limitations should be mentioned. First, the NHIS database does not include biochemical, imaging, or pathological information. Therefore, due to these pitfalls in the database characteristics, the operational definitions based on the diagnostic code, the number of laboratory tests, and surgery may not reflect the true diagnosis. Definitions based on the diagnostic code, surgery, or prescription code had to be assumed. The prevalence or incidence could therefore have been overestimated or underestimated. To overcome this limitation, the prevalence of PPGLs in a specific region was compared with that of PPGLs at the associated local hospital, which revealed a similar number of PPGL patients. Parasympathetic PGLs can be non-functional, and these may have not been included in the present study. Finally, only patients who underwent surgery were included, so some patients who did not undergo surgery may have been missed. The causes of death could not be investigated since, due to an anonymity protocol, they were not accessible.
Collectively, the prevalence and annual incidence rates of PPGLs in Korea are 0.18 and 2.13 per 100,000 persons, respectively. Metastatic PPGLs accounted for 17.7% of all PPGLs, including 9.0% with metastasis at the time of diagnosis. PPGLs are accompanied by high rates of mortality and complications. This epidemiological study may pave the way for further research on PPGLs.
ACKNOWLEDGMENTS
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KNIDI), funded by the Ministry of Health and Welfare of the Republic of Korea (grant number: HI17C1926). We would like to thank the Committee of Clinical Practice Guidelines of the Korean Endocrine Society.
References
1. Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab (Seoul). 2017; 32:152–161. PMID: 28685506.

2. Plouin PF, Duclos JM, Soppelsa F, Boublil G, Chatellier G. Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: analysis of 165 operations at a single center. J Clin Endocrinol Metab. 2001; 86:1480–1486. PMID: 11297571.

3. Khorram-Manesh A, Ahlman H, Nilsson O, Oden A, Jansson S. Mortality associated with pheochromocytoma in a large Swedish cohort. Eur J Surg Oncol. 2004; 30:556–559. PMID: 15135486.

4. Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011; 29:2049–2060. PMID: 21826022.

5. Zelinka T, Petrak O, Turkova H, Holaj R, Strauch B, Krsek M, et al. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res. 2012; 44:379–384. PMID: 22517556.

6. Kim KY, Kim JH, Hong AR, Seong MW, Lee KE, Kim SJ, et al. Disentangling of malignancy from benign pheochromocytomas/paragangliomas. PLoS One. 2016; 11:e0168413. PMID: 27992508.

7. Plouin PF, Fitzgerald P, Rich T, Ayala-Ramirez M, Perrier ND, Baudin E, et al. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm Metab Res. 2012; 44:390–399. PMID: 22314389.

8. Hong AR, Kim JH, Park KS, Kim KY, Lee JH, Kong SH, et al. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. Eur J Endocrinol. 2017; 177:475–483. PMID: 28870984.

9. Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000; 85:637–644. PMID: 10690869.
10. McNeil AR, Blok BH, Koelmeyer TD, Burke MP, Hilton JM. Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Aust N Z J Med. 2000; 30:648–652. PMID: 11198571.

11. Berends AMA, Buitenwerf E, de Krijger RR, Veeger NJGM, van der Horst-Schrivers ANA, Links TP, et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: a nationwide study and systematic review. Eur J Intern Med. 2018; 51:68–73. PMID: 29361475.

12. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017; 46:799–800. PMID: 27794523.

13. Sinclair AM, Isles CG, Brown I, Cameron H, Murray GD, Robertson JW. Secondary hypertension in a blood pressure clinic. Arch Intern Med. 1987; 147:1289–1293. PMID: 3606286.

14. Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994; 12:609–615. PMID: 7930562.

15. Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000; 6:249–252. PMID: 11421540.

16. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004; 27:193–202. PMID: 15080378.

17. Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983; 58:802–804. PMID: 6645626.
18. Guerrero MA, Schreinemakers JM, Vriens MR, Suh I, Hwang J, Shen WT, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009; 209:727–732. PMID: 19959041.

19. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017; 23:999–1005. PMID: 28613940.

20. Beninato T, Kluijfhout WP, Drake FT, Lim J, Kwon JS, Xiong M, et al. Resection of pheochromocytoma improves diabetes mellitus in the majority of patients. Ann Surg Oncol. 2017; 24:1208–1213. PMID: 27896511.

21. Kim BJ, Kwak MK, Ahn SH, Kim H, Lee SH, Song KH, et al. Lower bone mass and higher bone resorption in pheochromocytoma: importance of sympathetic activity on human bone. J Clin Endocrinol Metab. 2017; 102:2711–2718. PMID: 28582552.

22. Kim BJ, Lee SH, Koh JM. Bone health in adrenal disorders. Endocrinol Metab (Seoul). 2018; 33:1–8. PMID: 29589383.

23. Hamidi O, Young WF Jr, Iniguez-Ariza NM, Kittah NE, Gruber L, Bancos C, et al. Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab. 2017; 102:3296–3305. PMID: 28605453.

24. Choi YM, Sung TY, Kim WG, Lee JJ, Ryu JS, Kim TY, et al. Clinical course and prognostic factors in patients with malignant heochromocytoma and paraganglioma: a single institution experience. J Surg Oncol. 2015; 112:815–821. PMID: 26464058.
25. Li ML, Fitzgerald PA, Price DC, Norton JA. Iatrogenic pheochromocytomatosis: a previously unreported result of laparoscopic adrenalectomy. Surgery. 2001; 130:1072–1077. PMID: 11742341.

26. Bosca Robledo A, Ponce Marco JL, Belda Ibanez T, Meseguer Anastasio MF, Gomez Gavara C. Pheochromocytomatosis: a risk after pheochromocytoma surgery. Am Surg. 2010; 76:E122–E124. PMID: 21513631.
27. Hamidi O, Young WF Jr, Gruber L, Smestad J, Yan Q, Ponce OJ, et al. Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2017; 87:440–450. PMID: 28746746.

28. Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011; 96:717–725. PMID: 21190975.

29. Goffredo P, Sosa JA, Roman SA. Malignant pheochromocytoma and paraganglioma: a population level analysis of long-term survival over two decades. J Surg Oncol. 2013; 107:659–664. PMID: 23233320.

Fig. 2
(A) Age and (B) sex distributions of study subjects with pheochromocytomas and paragangliomas. The sex of three subjects was not identified.
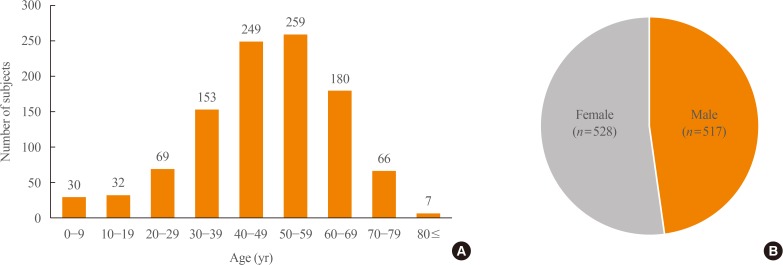
Fig. 3
The number of study subjects and the incidence rate of pheochromocytomas and paragangliomas by year.
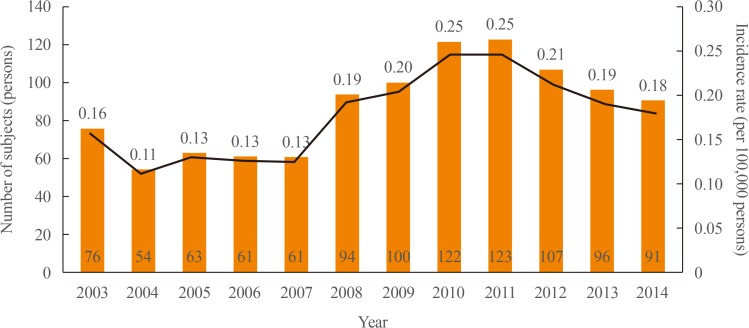
Fig. 4
Kaplan-Meier survival curve for metastasis in non-metastatic pheochromocytomas and paragangliomas at diagnosis.
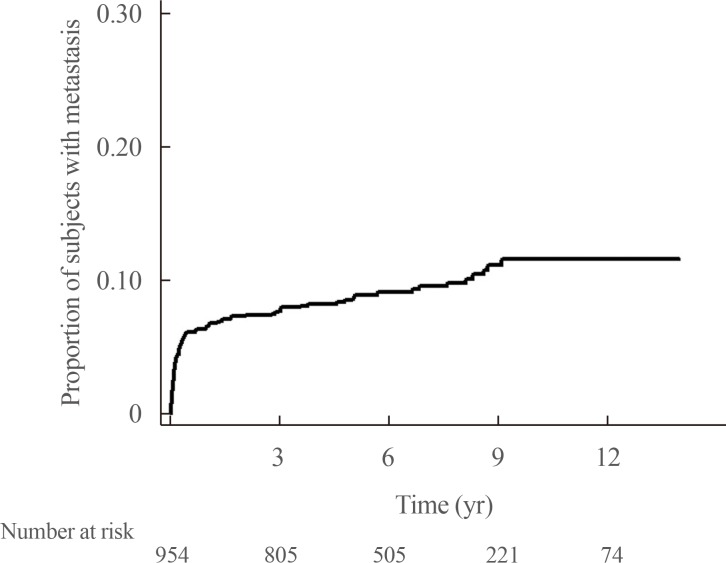
Fig. 5
Kaplan-Meier survival curve according to metastasis at diagnosis. PPGL, pheochromocytomas and paraganglioma.
Table 1
Prevalence of Comorbidities among Study Subjects (n=1,048)
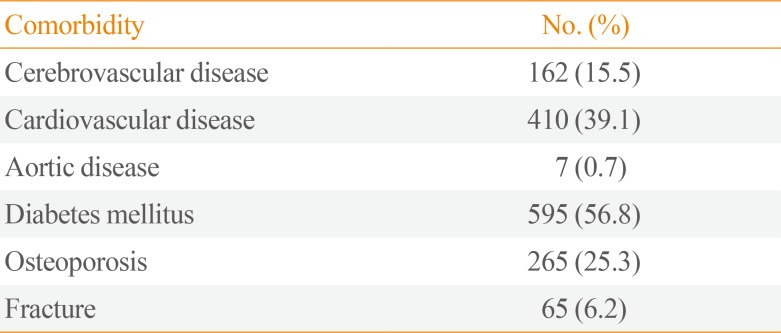
| Comorbidity | No. (%) |
|---|---|
| Cerebrovascular disease | 162 (15.5) |
| Cardiovascular disease | 410 (39.1) |
| Aortic disease | 7 (0.7) |
| Diabetes mellitus | 595 (56.8) |
| Osteoporosis | 265 (25.3) |
| Fracture | 65 (6.2) |
Table 2
HRs for Mortality in Subjects with Metastatic PPGLs at Any Time (n=185) Compared to Those with Nonmetastatic PPGLs (n=863)
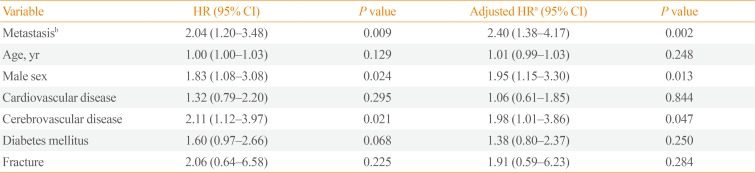
HR, hazard ratio; PPGL, pheochromocytomas and paraganglioma; CI, confidence interval.
aAdjusted for age, sex, cerebrovascular disease, cardiovascular disease, diabetes mellitus, and fractures; bMetastasis at initial diagnosis or during follow-up. HRs compared with subjects with non-metastatic PPGLs are shown.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download