Abstract
Background
To evaluate the imaging features, clinical manifestations, and prognosis of patients with thyroid nodule rupture after radiofrequency ablation (RFA).
Methods
The records of 12 patients who experienced thyroid nodule rupture after RFA at four Korean thyroid centers between March 2010 and July 2017 were retrospectively reviewed. Clinical data evaluated included baseline patient characteristics, treatment methods, initial presenting symptoms, imaging features, treatment, and prognosis.
Results
The most common symptoms of post-RFA nodule rupture were sudden neck bulging and pain. Based on imaging features, the localization of nodule rupture was classified into three types: anterior, posterolateral, and medial types. The anterior type is the most often, followed by posterolateral and medial type. Eight patients recovered completely after conservative treatment. Four patients who did not improve with conservative management required invasive procedures, including incision and drainage or aspiration.
Conclusion
Thyroid nodule rupture after RFA can be classified into three types based on its localization: anterior, posterolateral, and medial types. Because majority of thyroid nodule ruptures after RFA can be managed conservatively, familiarity with these imaging features is essential in avoiding unnecessary imaging workup or invasive procedures.
Radiofrequency ablation (RFA) is a minimally invasive technique that has been used to treat various tumors and has been shown effective in patients with benign thyroid nodules [1234] and recurrent thyroid cancers [567]. Although RFA is relatively safe for treating thyroid nodules, as shown in several systematic reviews and meta-analyses [8910], it is technically challenging and requires extensive experience [1011]. Understanding all possible complications of RFA is required for proper diagnosis and treatment.
The most common major complication after RFA is voice change [810], caused by injury to the recurrent laryngeal or vagus nerve, but this complication may be reduced by the use of a trans-isthmic approach and moving shot technique during RFA [8910]. The second most common major complication is nodule rupture after RFA [810]. To date, however, little is known about the imaging features, clinical course, and risk factors for this adverse event [8121314]. This study therefore evaluated the imaging features, clinical manifestations, and prognosis of patients with thyroid nodule rupture after RFA.
The Institutional Review Boards of the four participating thyroid centers approved the protocol of this retrospective study (S2017-1436-0001). All patients provided written informed consent prior to RFA.
The records of 12 patients who experienced thyroid nodule rupture after RFA at four Korean thyroid centers between March 2010 and July 2017 were retrospectively reviewed. Clinical data included baseline patient characteristics, treatment method, initial presenting symptoms, imaging features, treatment, and prognosis were evaluated.
Before RFA, all nodules were found to be benign by fine needle aspiration or core needle biopsy [151617]. Ultrasound (US)-guided RFA was performed by the same radiologist who performed the US assessment. All ablations were performed using a radiofrequency (RF) generators (Cool-Tip RF system, Covidien, Boulder, CO, USA; SSP-2000, Taewoong Medical, Gimpo, Korea; and M-1004, RF Medical, Seoul, Korea) and an 18-gauge internally cooled electrode (Well-Point RF Electrodes, Taewoong Medical, Goyang, Korea; VIVA, STARmed, Goyang, Korea; Big-Tip, RF Medical, Seoul, Korea; Cool-tip RF system, Radionics, Valleylab, Boulder, CO, USA) with 0.7-, 1-, 1.5-, and 2-cm active tips, respectively, depending on the size of the nodule and the preference of the radiologists. Local anesthesia consisted of injection of 2% lidocaine into the puncture site and perithyroidal area [18]. RFA was performed using the trans-isthmic approach and moving shot technique under US guidance [2411]. If nodules contained fluid, the fluid was aspirated prior to RFA [1920]. Adjacent structures, including nerves and vessels, were carefully monitored during the procedure to avoid complications [21]. The electrode tip was initially positioned in the deepest and farthest area of the nodule and then moved after a transient hyperechoic zone appeared within 5 to 10 seconds of turning on the RF power [2411]. If a hyperechoic microbubble did not form at the electrode tip within 5 to 10 seconds, the RF power was increased in 10 W increments. If the patient complained of pain during the procedure, the RF power was reduced or turned off for several seconds. The procedure was terminated when the entire visualized area of the nodule had become a transient hyperechoic zone. Repeat ablations were performed when unsatisfactory volume reductions (50%) of treated nodules after 3 months and the presence of an initially untreated portion [12]. All patients suspected of nodule rupture after RFA were examined by US and/or computed tomography (CT).
A review of medical records identified 12 patients, three men and nine women, of mean age 41 years (range, 16 to 75), who experienced post-RFA nodule rupture. Their clinical details are summarized in Table 1. Of these 12 patients, 10 had thyroid nodules with solid and cystic portions and two had solid nodule. The mean volume of the index nodules was 17.0 mL (range, 0.19 to 74.6). One patient required three RFA procedures, two required two procedures, and nine required one ablation. The mean ablation time and mean maximal ablation power per RFA session were 13.4 minutes (range, 4.7 to 40) and 57.5 W (range, 30 to 110), respectively.
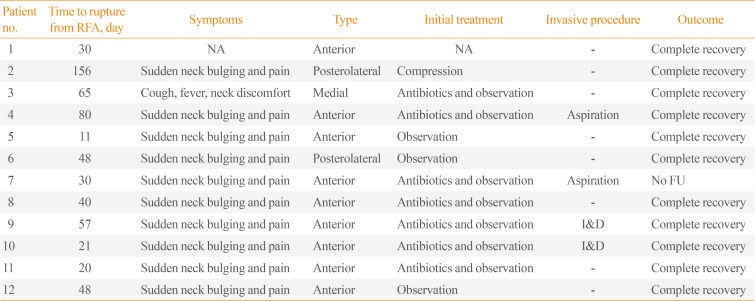
Table 2 summarizes the clinical and imaging manifestations of these 12 patients with thyroid nodule rupture. The most common symptoms of post-RFA nodule rupture during follow-up were sudden neck bulging and pain. Only one patient reported fever, cough, and neck discomfort. The mean time from RFA to nodule rupture was 54.6 days (range, 11 to 156).
Following the development of symptoms, 10 patients were evaluated by US, one by CT, and one by US and CT. Based on their localization on imaging features, nodule rupture could be classified into three types, the most common feature being anterior type, observed in nine patients (75%) and characterized by extension of the nodule contents into the anterior extrathyroidal area and sometimes into the strap muscle, with discontinuity of the anterior thyroid capsule (Fig. 1). Two patients (16.7%) had the posterolateral type of nodule rupture, which was characterized by heterogeneous fluid collection around the thyroid capsule (Fig. 2). One patient (8.3%) had the medial type of rupture, which was characterized on CT by soft tissue bulging at the medial aspect of the ablated nodule and protrusion into the lumen of the trachea (Fig. 3). This patient experienced fever, cough, and neck discomfort, unlike the other patients.
All 12 patients were initially treated conservatively, with close observation and/or oral antibiotics. Eight patients recovered completely with conservative treatment after mean follow-up period of 71 days (range, 11 to 202). The mean period of use of antibiotics was 16 days (range, 7 to 34). Four patients required invasive procedures, including incision and drainage or aspiration. The nature of aspirated fluid were hemorrhage in two patients and pus in two patients. All of these four patients had the anterior type of rupture. Fig. 1 shows the US images of one patient who underwent aspiration. Sonography showed breakdown of the anterior thyroid capsule and the communication between intra- and extrathyroidal lesions at the RF site. US also showed a heterogeneous mass-like lesion extending from the thyroid nodule to the superficial aspect of the strap muscle, with Doppler US showing increased vascularity. US-guided core needle biopsy showed that the mass-like lesion was an organizing hematoma. Despite compression, this hematoma increased in size on follow-up US. US-guided aspiration resulted in the removal of dark blood material, with follow-up US showing that this patient recovered completely. Including this patient, all patients, except for the one lost to follow-up, experienced complete recovery.
This multicenter study, which describes 12 patients who experienced thyroid nodule rupture after RFA, is, to our knowledge, the first original study to classify types of post-RFA nodule rupture according to their localization. The anterior type is the most often, followed by posterolateral and medial type. Despite differences in their imaging features, 67% patients (eight of 12 patients) with thyroid nodule ruptures were successfully managed conservatively. Knowledge about the typical clinical symptoms (sudden neck bulging and pain) and imaging features of post-RFA nodule rupture can ensure the proper diagnosis of patients and reduce unnecessary treatment.
RFA is a minimally invasive technique that may serve as a safe and effective alternative to surgery in managing benign thyroid nodules that cause symptoms and cosmetic problems [1234222324252627]. A recent meta-analysis indicated that the mean volume change following RFA for benign thyroid nodules was 76.1% (95% confidence interval [CI], 70.1% to 82.1%), with an absolute mean change in volume of 8.9 mL (95% CI, 6.6 to 11.2) [27]. RFA is also reported to improve symptom and cosmetic scores, as well as having low complication rates [123222324]. Although RFA has a lower complication rate than surgery, complications can still occur [89101328], with nodule rupture being the second most common major complication [810]. Thyroid nodule rupture after RFA is defined as the breakdown of the thyroid capsule and the communication between intra- and extrathyroidal lesions. Thyroid nodule rupture has firstly been reported after laser ablation [29]. In that study, three patients had pseudocysts with fasciitis owing to fluid leakage in the neck muscle fascia, followed by spontaneous reabsorption of these at 3 to 6 months. A subsequent report described six patients at four institutions who experienced thyroid nodule rupture after RFA [12], and there have been occasional case reports describing the occurrence of nodule rupture [1314].
All previous ruptures that could be evaluated using imaging modalities were anterior type [812]. However, although our review of 12 patients at four institutions found that the anterior type was the most common, two patients had the posterolateral type and one had the medial type of rupture. The anterior type typically exhibits an anterior bulging of a mass that can extend to the strap muscle layer. The posterolateral type is a hyperechoic lesion that surrounds the thyroid capsule rather than a bulging anterior mass. The medial type shows a discontinuity of the medial thyroid capsule, with the internal contents bulging toward the tracheoesophageal (TE) groove, causing a mass effect on the trachea. Because the TE groove area is a relatively limited space, unlike the anterior or posterolateral area, the medial rupture is the most uncommon type and its causes remain unclear. Familiarity with these imaging characteristics is important in distinguishing among these types of nodule rupture after RFA and in avoiding additional unnecessary imaging workup or invasive procedures. In fact, the one patient with the posterolateral type and one with the medial type were referred to tertiary referral center from local clinics because of misdiagnosis.
Thyroid nodule rupture after RFA is thought to be caused by delayed bleeding, as shown by the high attenuation observed on CT with volume expansion of treated nodule, as well as aspiration of old blood in all patients who underwent invasive procedures. Bleeding may result from microvascular leakage within the tumor, leading to delayed volume expansion and rupture; tearing of the tumor wall and thyroid capsule at a weak point; or post-procedural massaging or moving of the neck. The differences in imaging features observed in the three types of post-RFA nodule rupture may arise from differences in the causes of rupture. The anterior type may be caused by a more acute expansion of the treated nodule with hemorrhage, whereas the posterolateral type may be caused by a more persistent oozing. All four patients who required invasive procedures had the anterior type of rupture, perhaps because this type is caused by a relatively large volume of hemorrhage. Because hemorrhage is basically aseptic and self-limiting, we recommend that these patients should be managed conservatively with observation and compression, with or without oral antibiotics, rather than undergo invasive procedures [12]. Eight of the 12 patients in this study completely recovered without any invasive procedure, such as aspiration or drainage. Invasive procedures should be performed only when conservative management is unsuccessful.
In conclusion, thyroid nodule rupture after RFA can be classified into three types based on its localization: anterior, posterolateral, and medial types. Because majority of thyroid nodule ruptures after RFA can be managed conservatively, familiarity with these imaging features is essential in avoiding unnecessary imaging workup or invasive procedures.
References
1. Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009; 33:1971–1977. PMID: 19575141.

2. Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008; 18:1244–1250. PMID: 18286289.

3. Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006; 16:361–367. PMID: 16646682.

4. Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010; 194:1137–1142. PMID: 20308523.

5. Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011; 197:W331–W336. PMID: 21785061.

6. Guenette JP, Monchik JM, Dupuy DE. Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J Vasc Interv Radiol. 2013; 24:672–679. PMID: 23622038.

7. Kim JH, Yoo WS, Park YJ, Park DJ, Yun TJ, Choi SH, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015; 276:909–918. PMID: 25848897.

8. Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012; 262:335–342. PMID: 21998044.

9. Wang JF, Wu T, Hu KP, Xu W, Zheng BW, Tong G, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J (Engl). 2017; 130:1361–1370. PMID: 28524837.
10. Chung SR, Suh CH, Baek JH, Park HS, Choi YJ, Lee JH. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017; 33:920–930. PMID: 28565997.

11. Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018; 19:632–655. PMID: 29962870.

12. Shin JH, Jung SL, Baek JH, Kim JH. Rupture of benign thyroid tumors after radio-frequency ablation. AJNR Am J Neuroradiol. 2011; 32:2165–2169. PMID: 21920870.

13. Che Y, Jin S, Shi C, Wang L, Zhang X, Li Y, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015; 36:1321–1325. PMID: 25814656.

14. Valcavi R, Tsamatropoulos P. Health-related quality of life after percutaneous radiofrequency ablation of cold, solid, benign thyroid nodules: a 2-year follow-up study in 40 patients. Endocr Pract. 2015; 21:887–896. PMID: 26121459.

15. Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017; 18:217–237. PMID: 28096731.

16. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–395. PMID: 27134526.

17. Baek JH. Current status of core needle biopsy of the thyroid. Ultrasonography. 2017; 36:83–85. PMID: 28301922.

18. Park HS, Baek JH, Park AW, Chung SR, Choi YJ, Lee JH. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017; 18:615–623. PMID: 28670156.

19. Park HS, Baek JH, Choi YJ, Lee JH. Innovative techniques for image-guided ablation of benign thyroid nodules: combined ethanol and radiofrequency ablation. Korean J Radiol. 2017; 18:461–469. PMID: 28458598.

20. Sim JS, Baek JH, Lee J, Cho W, Jung SI. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017; 33:905–910. PMID: 28540795.

21. Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015; 16:749–766. PMID: 26175574.

22. Deandrea M, Limone P, Basso E, Mormile A, Ragazzoni F, Gamarra E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008; 34:784–791. PMID: 18207307.

23. Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010; 34:1488–1493. PMID: 20376445.

24. Spiezia S, Garberoglio R, Milone F, Ramundo V, Caiazzo C, Assanti AP, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009; 19:219–225. PMID: 19265492.

25. Hong MJ, Baek JH, Choi YJ, Lee JH, Lim HK, Shong YK, et al. Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. J Vasc Interv Radiol. 2015; 26:55–61. PMID: 25446422.
26. Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013; 23:1044–1049. PMID: 23096937.

27. Ha EJ, Baek JH, Kim KW, Pyo J, Lee JH, Baek SH, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and Bayesian network meta-analysis. J Clin Endocrinol Metab. 2015; 100:1903–1911. PMID: 25695887.

28. Bernardi S, Dobrinja C, Fabris B, Bazzocchi G, Sabato N, Ulcigrai V, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014; 2014:934595. PMID: 25045352.

29. Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010; 20:1253–1261. PMID: 20929405.

Fig. 1
(A) A 67-year-old man, who presented with a bulging neck mass, had been treated with radiofrequency ablation (RFA) due to a predominantly solid thyroid nodule at right thyroid gland. He had sudden bulging and pain on the right side of his neck 80 days after the RFA. (B, C, D) Computed tomography (CT) and (E, F) ultrasound show volume expansion and discontinuity of the anterior thyroid capsule with nodule content extended to the anterior extra-thyroidal area (arrows) (anterior type). (D) Intra-nodular hyper-attenuating portions on pre-contrast CT represent intra-nodular bleeding. (G) The patient was managed with fluid aspiration and intravenous antibiotics, and the lesion gradually regressed.
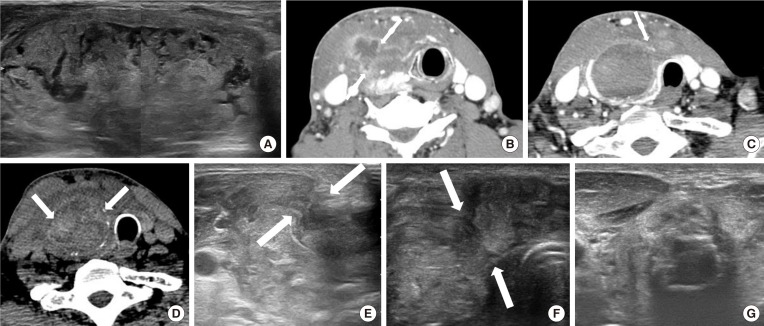
Fig. 2
(A) A 24-year-old woman, who presented with a bulging neck mass, had been treated with radiofrequency ablation (RFA) due to a predominantly solid thyroid nodule at right thyroid gland. (B) One month after RFA, the nodule decreased in size. However, at 156 days after RFA, the patient complained of neck pain and bulging. (C) Ultrasound shows a heterogeneous echoic lesion around the thyroid capsule (arrows) (posterolateral type). She was treated with compression and observation. (D) After a month, the lesion was completely disappeared.
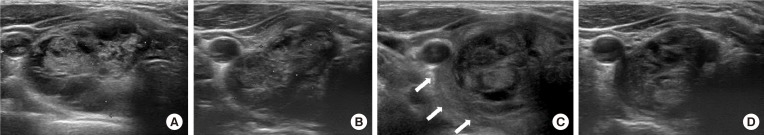
Fig. 3
(A) A 42-year-old female, who presented with bulging neck mass, had been treated with radiofrequency ablation (RFA) due to a predominantly solid thyroid nodule at right thyroid gland. She complained of cough, neck discomfort and mild fever 65 days after RFA. (B, C) On computed tomography (CT) scan, the ablated nodule bulges to medial side and protrudes into the tracheal lumen (arrows) (medial type). (D, E) After conservative management with antibiotics, the lesion was completely disappeared on follow-up CT and ultrasound after 2 months.
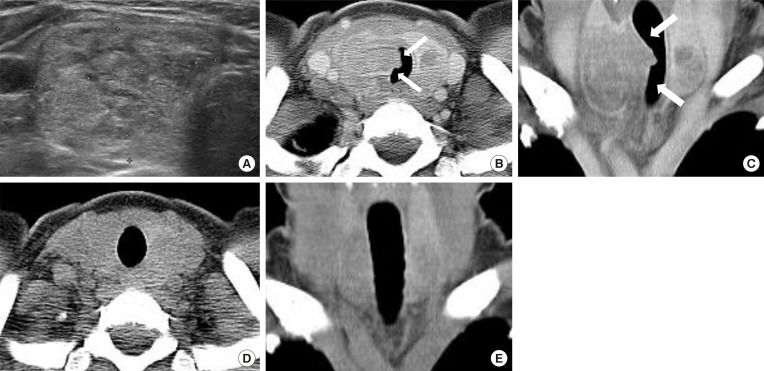
Table 1
Baseline Characteristics of the 12 Patients with Thyroid Nodule Rupture after RFA
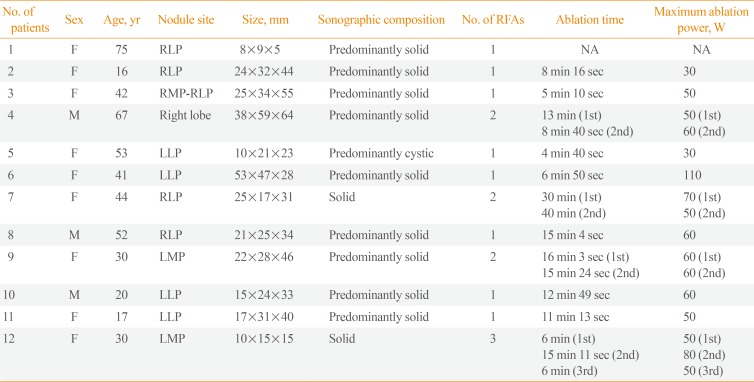
Table 2
Clinical and Imaging Manifestations in the 12 Patients with Thyroid Nodule Rupture after RFA




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download