Abstract
Background
Although previous studies have demonstrated that irisin plays an anti-inflammatory role in the body, conflicting results have been reported regarding the correlation between serum levels of irisin and C-reactive protein (CRP). The present meta-analysis was conducted to further investigate the correlation between irisin and CRP levels.
Methods
We systematically searched PubMed, the Cochrane Library, Web of Science, Embase, SCOPUS, and Ovid to retrieve studies assessing the correlation between irisin and CRP levels. Meta-analyses were performed using a random-effects model, and the I2 index was used to evaluate heterogeneity.
Results
Of the 428 studies that were initially found, 14 studies with 2,530 participants met the inclusion criteria for the meta-analysis. The pooled effect size was calculated as 0.052 (95% confidence interval, −0.047 to 0.152; P=0.302). Subgroup analyses identified s ignificant, positive, but weak correlations between CRP and irisin levels in cohort studies, studies conducted among healthy participants, studies in which the male-to-female ratio was less than 1, in overweight or obese subjects, and in studies with a sample size of at least 100 participants.
Irisin, a novel peptidic myokine, was discovered by Bostrom et al. [1] in 2012. It is secreted from skeletal muscle tissue through the cleavage of fibronectin type III domain containing 5 (FNDC5), and is regulated by peroxisome proliferator-activated receptor (PPAR)-coactivator-1α (PGC-1α) [1]. Irisin stimulates thermogenesis via browning of white adipose tissue, and subsequently increases energy expenditure, improves glucose homeostasis, and regulates energy metabolism [2]. In addition to its regulatory role in metabolism, a limited number of studies have demonstrated that it plays a role in immunity, and most of those studies found that irisin has anti-inflammatory properties [3]. In light of its function in metabolism and immunity, irisin has been considered as a new therapeutic target for metabolic disorders [456].
C-reactive protein (CRP), a common acute-phase reactant, is a sensitive serum parameter for diagnosing systemic inflammation [789]. It has been well documented that pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β, can regulate the production and secretion of CRP [10]. In response to any inflammatory stimulus, serum levels of CRP increase and then decrease very rapidly [10]. Moreover, it has been shown that serum levels of CRP and other inflammatory biomarkers are influenced by muscle contraction, as well as by serum irisin levels [11]. This finding implies a relationship between serum concentrations of irisin and CRP. The possible association between irisin and CRP has been assessed in several studies. Hou et al. [12] reported that circulating irisin was significantly and negatively correlated with high-sensitivity CRP. However, another study found a significant positive correlation between irisin and high-sensitivity CRP levels in the general population [13]. In another study conducted by Jameel et al. [14], no significant correlation was seen between serum levels of irisin and high-sensitivity CRP in healthy adults. Due to the inconsistent results of previous studies, we carried out a systematic review and meta-analysis of extant observational studies to assess the correlation between circulating irisin levels and serum levels of CRP.
A comprehensive literature search of six databases, including PubMed, the Cochrane Library, Web of Science, Embase, SCOPUS, and Ovid, was conducted using the keywords “C reactive protein,” “C-reactive protein,” “high-sensitivity CRP,” “hs-CRP,” “high sensitivity CRP,” “hscrp,” and “CRP” in combination with the keywords “irisin” and “FNDC5” for studies in all languages published up to March 28, 2018. The complete search strategy is presented in Supplemental File S1.
All the potentially relevant studies obtained from the databases were reviewed by two investigators (E.E. and M.Z.K.). The titles and abstracts of retrieved publications were initially screened for potentially eligible studies, which were subsequently evaluated by full-text review. Studies were included if the study population was adults (mean age ≥18 years) and if they presented results on the relationship between circulating irisin and CRP levels (measured in plasma or serum). All types of studies except animal-based studies, reviews, posters, and letters to the editor were included. Studies were excluded if the participants were lactating or pregnant woman and if complete data were unavailable. The initial search was supplemented by checking the reference lists of the retrieved articles to identify missed studies. Disagreements about the eligibility of any article were solved by discussing with a third author (A.A.). This study was exempted from the requirement for a consent form.
Data from eligible studies were extracted by two investigators (E.E. and O.A.) using an Excel form. The following data were extracted from each eligible study: first author, publication year, study location, study population, study design, sample size, type of blood sample, circulating irisin and CRP levels, age, body mass index (BMI), sex ratio, measures of association, and brief results together with the adjusted covariates. When the data were insufficient for a meta-analysis, we contacted the authors directly to obtain the data. Study quality was assessed by the Newcastle-Ottawa Scale (NOS) [15].
Meta-analysis was carried out using Stata version 12.0 (Stata Corp., College Station, TX, USA). A fixed-effects model was used for the assessment of the pooled effect size. When heterogeneity was present, a random-effects model was used. Heterogeneity was tested using the I2 statistic, and an I2 value ≥50% with a level of significance of P<0.05 by the Cochran Q-test was interpreted as evidence of substantial heterogeneity. Publication bias was assessed by a funnel plot analysis, the Begg adjusted rank correlation test, and the Egger regression asymmetry test. Subgroup analyses were carried out based on the quality of the studies, disease status of the participants, sex ratio (men vs. women), study design (cross-sectional vs. case-control vs. cohort), sample size, and BMI of the participants. The trim-and-fill method was used to explore the number and outcomes of potential missing studies. Additionally, the metaninf test was used to assess the effect of individual studies by estimating the r values obtained when each study was omitted.
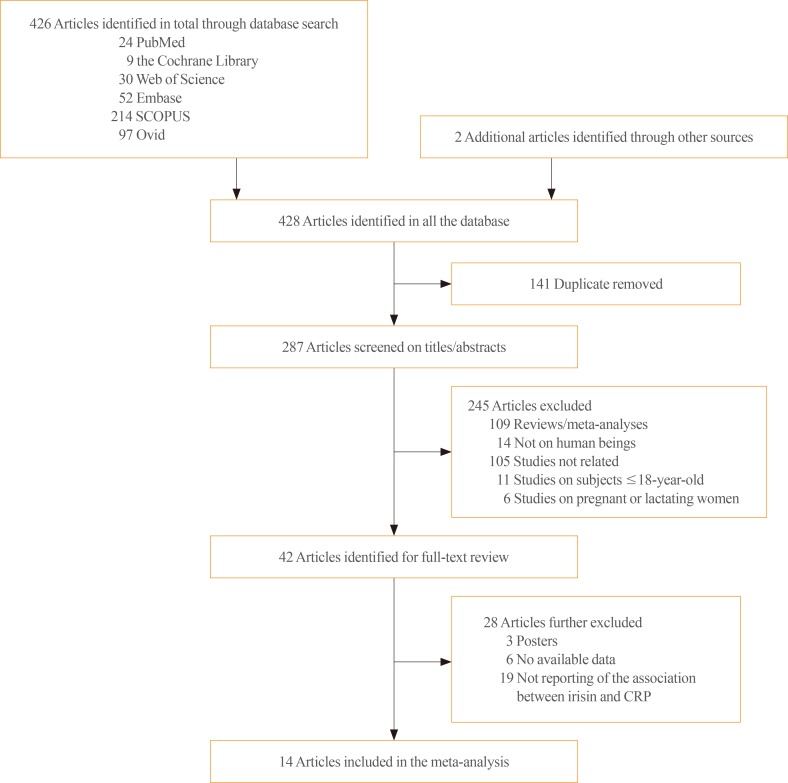
The first step of the search yielded 24, 9, 30, 52, 214, and 97 citations in PubMed, Cochrane Library, Web of Science, Embase, SCOPUS, and Ovid, respectively (Fig. 1). Two additional studies were found while searching the reference lists [1617]. Of these, 141 articles were excluded due to duplication. The titles and abstracts of 287 articles were reviewed. Forty-two studies were identified for full-text review. Of these, 28 studies were excluded for the following reasons: three studies were posters, data were not available for six studies, and 19 studies did not report the association between irisin and CRP levels. Ultimately, 14 articles were included in the meta-analysis [1213141617181920212223242526].
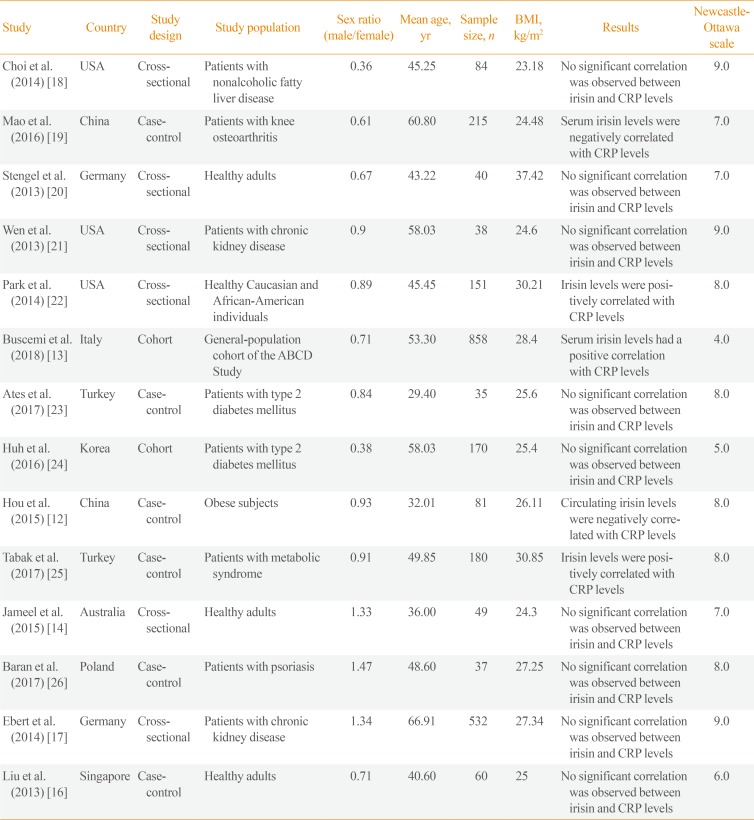
The selected studies were published between 2012 and 2017 (Table 1), with a total sample size of 2,530. The mean age of the participants ranged from 29 to 66.9 years, and the mean BMI ranged from 23.18 to 37.42 kg/m2. All studies included both sexes, and the ratio of male to female participants ranged from 0.36 to 1.47. Three studies were from the USA, and others were from China, Germany, Italy, Turkey, Korea, Australia, Poland, and Singapore. Six studies were cross-sectional studies, six were case-control studies, and two were cohort studies. Two of the studies included type 2 diabetes mellitus patients, five studies included healthy individuals or the general population, two studies included patients with chronic kidney disease, one study included patients with psoriasis, one study included patients with metabolic syndrome, one study included obese subjects, one study included nonalcoholic fatty liver disease patients, and one study included patients with knee osteoarthritis. Seven studies had sample sizes of 100 to 1,000 patients, while the remaining seven studies had a sample size under 100 patients. Four studies used the enzyme-linked immunosorbent assay (ELISA) method to determine the circulating levels of CRP, and seven studies used other methods, such as immunoturbidimetric assays. All studies used the ELISA method to evaluate circulating levels of irisin.
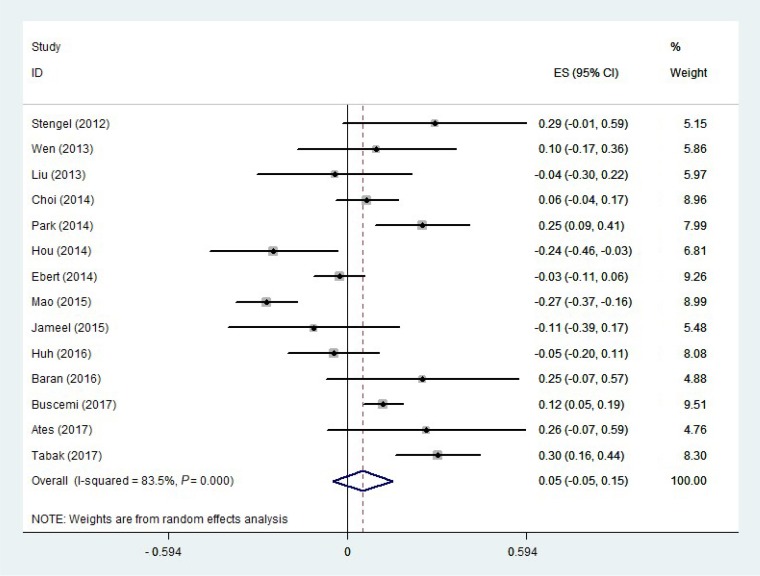
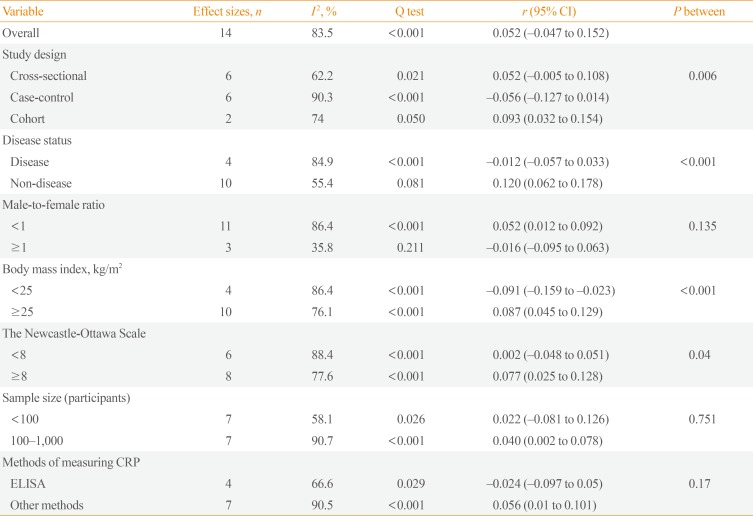
Of the 14 studies selected for the meta-analysis, nine reported no correlation between circulating irisin and CRP levels, three reported a positive correlation, and two reported a negative correlation. A random-effects model was used to estimate the overall correlation between irisin and CRP levels. The pooled effect size was calculated at 0.052 (95% confidence interval [CI], −0.047 to 0.152; P=0.302) (Fig. 2). There was a moderate-to-high level of heterogeneity (I2=83.5%, P<0.001) among the included studies. Therefore, to identify the sources of heterogeneity, we performed subgroup analyses. The subgroup analysis by study design indicated that heterogeneity was lower in both cross-sectional and cohort studies (I2=62.2%, P=0.021; I2=74.0%, P=0.050; respectively) (Table 2), and the correlation was only significant in cohort studies. The subgroup analysis by disease status demonstrated that disease status of the participants was a source of heterogeneity (Table 2). In the subgroup of healthy people and the general population, the heterogeneity was not significant (I2=55.4%, P=0.081) (Table 2). As shown in Table 2, a significant positive correlation between CRP and irisin levels was found in the subgroup of healthy people and the general population (r=0.12; 95% CI, 0.062 to 0.178). Another source of heterogeneity was the male-to-female ratio. As Table 2 indicates, heterogeneity was lower in studies in which the male-to-female ratio was 1 or higher (I2=35.8%, P=0.211). In addition, there was a significant direct correlation between CRP and irisin levels in studies where the male-to-female ratio was lower than 1 (Table 2). The subgroup analysis according to BMI indicated that a significant positive correlation was present in participants with a BMI ≥25 kg/m2 (Table 2). Moreover, as shown in Table 2, a significant but weak positive correlation was found between CRP and irisin levels in high-quality studies (NOS ≥8) (r=0.077; 95% CI, 0.025 to 0.128). We likewise found a significant but weak positive correlation (r=0.040; 95% CI, 0.002 to 0.078) in studies with a sample size of more than 100 participants (Table 2). Furthermore, the subgroup analysis according to the methods of measuring CRP indicated that there was no significant correlation in studies that used ELISA, whereas a significant positive correlation was found in studies that used other methods (Supplemental Fig. S1).
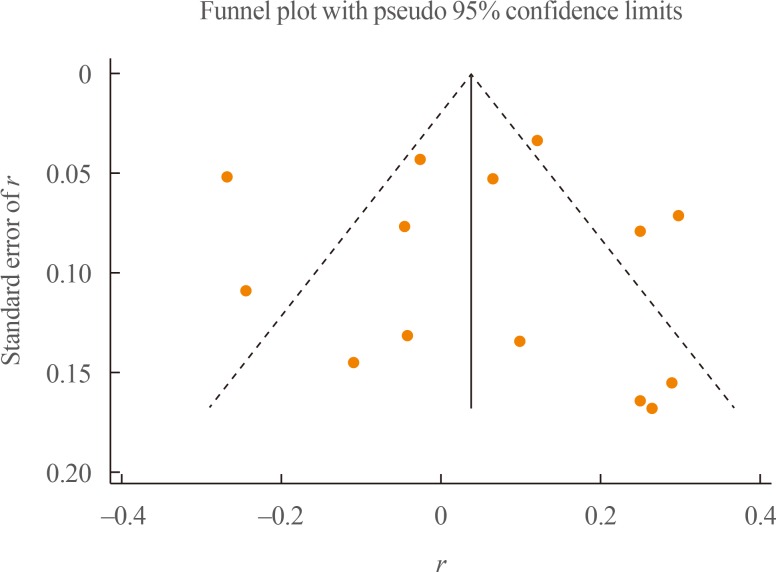
The evaluation of publication bias using funnel plots demonstrated no evidence of publication bias within the studies (Fig. 3). Furthermore, both the Egger test and the Begg test showed no evidence of publication bias (P=0.897 and P=0.584, respectively). The trim-and-fill analysis explored the potential theoretical existence of two hypothetical studies that were not published due to publication bias; however, the results obtained by considering those two hypothetical studies demonstrated no significant correlation (pooled effect size, 0.015; 95% CI, −0.083 to 0.114), which was highly similar to the results without these studies. In the metaninf analysis, one study [19] seemed to influence the results (Supplemental Fig. S2). By omitting this study, the direction of the correlation between irisin and CRP did not change; however, it became significant (pooled effect size, 0.082; 95% CI, 0.001 to 0.164).
To our best knowledge, this is the first meta-analysis to assess the correlation between circulating irisin and CRP levels. In the overall results of the present meta-analysis, the circulating concentration of irisin had no correlation with that of CRP. Due to the significant heterogeneity among the studies, subgroup analyses were performed, finding that the study design, disease status of participants, sample size, and the male-to-female ratio were the main sources of heterogeneity. The results of the subgroup analyses indicated that there were significant, positive, but weak correlations between CRP and irisin levels in cohort studies, studies conducted among healthy participants, studies with a male-to-female ratio less than 1, participants with a BMI ≥25 kg/m2, high-quality studies, and studies with a sample size of at least 100 participants.
Irisin is a peptide that has been reported to play beneficial and protective roles for a wide range of disorders, such as insulin resistance, obesity, and metabolic disorders [2728]. Although numerous studies have been conducted on irisin and its role in metabolism, the literature contains scant data regarding its impact on inflammatory processes. Identifying the detailed characteristics of irisin in inflammatory processes is an important step towards the development of therapeutic strategies, especially for low-grade inflammation generated by immune cells in adipose tissues [3]. Most studies assessing the role of irisin in immunity have reported it to show anti-inflammatory properties. Irisin has been reported to reduce the expression and secretion of pro-inflammatory cytokines, such as TNF-α and IL-6 [29]. The precise anti-inflammatory mechanism of irisin is unclear; however, recent studies demonstrated that irisin can inhibit the activation of the Toll-like receptor 4 (TLR4) and nuclear factor-κB pathways, which are the most important pathways for activation of the innate immune system [3].
CRP is a classical acute-phase protein that is synthesized in the liver [30]. IL-6 and IL-1 are the main stimuli of CRP synthesis, although other agents, such as corticosteroids, can also influence its synthesis [10]. In general, serum CRP concentrations are correlated with those of other inflammatory markers, such as IL-6 [31]. Its serum level rises and falls more rapidly than other inflammatory markers, making it a useful marker to follow the clinical course of a disease and response to a specific treatment [10]. In human blood, circulating CRP binds to white blood cells through Fc receptors, specially the FcγRIIa receptor, which increases the production of cytokines [32]. Previous investigations revealed that CRP can increase the production of both pro- and anti-inflammatory cytokines [10]. Tilg et al. [33] suggested that the net effect of CRP is anti-inflammatory through its role in stimulating IL-10 and IL-1RA.
The overall results of the present meta-analysis demonstrated no significant correlation between circulating CRP and irisin levels. Of note, the results of the metaninf analysis indicated that by omitting the study of Mao et al. [19], the correlation between irisin and CRP became significant, albeit weak. Furthermore, it should be noted that after omitting this study, there was still a direct correlation between irisin and CRP. The study of Mao et al. [19] was conducted among patients with knee osteoarthritis, and demonstrated that serum levels of irisin were lower in these patients. A possible reason for lower levels of irisin in this group of patients could be lower levels of physical activity due to pain related to osteoarthritis. It has been thoroughly documented that irisin is a muscle-derived peptide that is secreted from contracting skeletal muscles, and lower levels of physical activity have therefore been associated with lower serum irisin levels [3435]. In contrast, in osteoarthritis patients, the serum level of CRP increases due to the inflammatory nature of the disease [36]. These considerations could jointly explain the significant negative correlation between irisin and CRP levels that was found by Mao et al. [19]. Therefore, it could be speculated that the serum concentration of irisin is influenced more by physical activity than by inflammation, although further studies are needed to confirm this hypothesis.
Previous investigations have demonstrated that exercise provokes elevated levels of cytokines [37]. Those studies reported that skeletal muscles can express and produce different kinds of cytokines, such as IL-6, IL-8, and IL-15 [37]. Thus, IL-6 has been labeled as a contraction-regulated myokine, similar to irisin, which is known to be a muscle-derived protein [37]. An elevation in the serum level of IL-6 is the earliest cytokine response to muscle contraction and exercise [37]. Increased serum levels of IL-6 can stimulate the production and secretion of CRP from the liver, increasing the serum concentration of CRP by as much as 100-fold [38]. Therefore, it can be speculated that during muscle contractions, the serum levels of both CRP and irisin increase.
It is notable that most of the studies analyzed herein did not find a significant correlation between irisin and CRP levels. As discussed above, the serum level of irisin is more strongly influenced by physical activity than by inflammation, meaning that physical activity should be considered as an important confounder of the results of studies assessing serum levels of irisin. Almost all the studies that assessed the correlation between irisin and CRP levels were limited by not evaluating the physical activity of participants. Furthermore, the health status of the population studied (healthy or having a specific disease) is an important factor that might have affected the results. As the results of the subgroup analyses indicated, there was a significant positive correlation between irisin and CRP levels in the subgroup of healthy participants, but there was no significant correlation in the subgroup of participants with a disease. Of note, the comorbidities in the disease group were very diverse, including type 2 diabetes mellitus, chronic kidney disease, psoriasis, nonalcoholic fatty liver disease, and osteoarthritis; the different origins of these diseases may have influenced the possible correlation between CRP and irisin levels. Therefore, our results should not be considered conclusive, and further studies are needed.
A positive correlation was found between CRP and irisin levels in overweight or obese participants. Circulating levels of both CRP and irisin are highly dependent on body fat mass. It has been reported that plasma irisin levels were positively correlated with body fat mass [20]. In addition, body fat can influence the serum levels of CRP [39]. IL-6 and TNF-α are secreted by subcutaneous adipose tissue, which are the main stimuli of CRP synthesis [10]. The subgroup analysis also indicated a positive correlation between CRP and irisin in studies with a male-to-female ratio less than 1. Previous investigations reported that females have a higher amount of body fat mass than males [40]. In addition, it has been found that irisin and CRP levels were higher in females than males [4142]. It is also important to note that high-quality studies, cohort studies, and studies with a larger sample size demonstrated a significant positive correlation between CRP and irisin levels. A well-designed cohort study with a large sample size can provide powerful results. Because the exposure is identified before the outcome, cohort studies have an appropriate temporal framework for evaluating causality, and therefore have the potential to provide the strongest scientific evidence. Moreover, the results of the subgroup analysis indicated that there was no significant correlation between CRP and irisin levels in subgroup of studies that used the ELISA method to assess CRP, whereas a significant positive correlation was found in studies that used other methods. Although the ELISA method has advantages, several weaknesses of this method have been recognized. The results obtained from this method are largely dependent on the operator's skill and experience, antibody quality, and the kit manufacturer [43]. Therefore, these shortcomings can limit the ability of ELISA to obtain accurate results.
This study has limitations. First, we did not limit the systematic search to a particular disease or condition, which led to an increase in heterogeneity. However, the subgroup analysis found lower levels of heterogeneity in some of the subgroups. Second, the small sample size of the individual studies limits the strength of the conclusions of the present meta-analysis, although we nonetheless hope that this study will be helpful for future research.
In conclusion, the results of the present meta-analysis indicated that there was no significant correlation between irisin and CRP levels. However, the subgroup analyses found significant, positive, but weak correlations between CRP and irisin levels in cohort studies, studies conducted among healthy participants, and studies with a male-to-female ratio less than 1. Furthermore, we found that there was a significant positive correlation between irisin and CRP in overweight or obese subjects, although it should be noted that there was a moderate-to-high level of heterogeneity between the studies in this subgroup. Despite the incremental impact of muscle contraction on the serum levels of both irisin and CRP, most of the studies included in this analysis did not assess the physical activity of the participants, which is an important limitation of those studies. Therefore, further well-designed studies are needed to confirm the results of the present meta-analysis.
References
1. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–468. PMID: 22237023.

2. Chen JQ, Huang YY, Gusdon AM, Qu S. Irisin: a new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015; 14:2. PMID: 25588692.

3. Mazur-Bialy AI, Pochec E, Zarawski M. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 2017; 18:E701. PMID: 28346354.

4. Raschke S, Eckardt K, Bjorklund Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS One. 2013; 8:e62008. PMID: 23637948.

5. Xiang L, Xiang G, Yue L, Zhang J, Zhao L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis. 2014; 235:328–333. PMID: 24911636.

6. Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, et al. Irisin in metabolic diseases. Endocrine. 2018; 59:260–274. PMID: 29170905.

7. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008; 9:46–56. PMID: 18073775.

8. Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83(6 Suppl):1505S–1519S. PMID: 16841861.

9. Li W, Luo X, Liu Z, Chen Y, Li Z. Prognostic value of C-reactive protein levels in patients with bone neoplasms: a meta-analysis. PLoS One. 2018; 13:e0195769. PMID: 29668751.

10. Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004; 30:261–277. PMID: 15531769.

11. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005; 45:1563–1569. PMID: 15893167.
12. Hou N, Han F, Sun X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin Endocrinol (Oxf). 2015; 83:339–343. PMID: 25382211.

13. Buscemi S, Corleo D, Vasto S, Buscemi C, Massenti MF, Nuzzo D, et al. Factors associated with circulating concentrations of irisin in the general population cohort of the ABCD study. Int J Obes (Lond). 2018; 42:398–404. PMID: 29027533.

14. Jameel F, Thota RN, Wood LG, Plunkett B, Garg ML. Sex-dependent association between circulating irisin levels and insulin resistance in healthy adults. J Nutr Intermed Metab. 2015; 2:86–92.

15. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute;c2019. cited 2019 Mar 28. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
16. Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. 2013; 27:365–369. PMID: 23619195.

17. Ebert T, Focke D, Petroff D, Wurst U, Richter J, Bachmann A, et al. Serum levels of the myokine irisin in relation to metabolic and renal function. Eur J Endocrinol. 2014; 170:501–506. PMID: 24399249.

18. Choi ES, Kim MK, Song MK, Kim JM, Kim ES, Chung WJ, et al. Association between serum irisin levels and non-alcoholic fatty liver disease in health screen examinees. PLoS One. 2014; 9:e110680. PMID: 25343462.

19. Mao Y, Xu W, Xie Z, Dong Q. Association of irisin and CRP levels with the radiographic severity of knee osteoarthritis. Genet Test Mol Biomarkers. 2016; 20:86–89. PMID: 26625129.

20. Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity: correlation with body mass index. Peptides. 2013; 39:125–130. PMID: 23219488.
21. Wen MS, Wang CY, Lin SL, Hung KC. Decrease in irisin in patients with chronic kidney disease. PLoS One. 2013; 8:e64025. PMID: 23667695.

22. Park KH, Zaichenko L, Peter P, Davis CR, Crowell JA, Mantzoros CS. Diet quality is associated with circulating C-reactive protein but not irisin levels in humans. Metabolism. 2014; 63:233–241. PMID: 24315778.

23. Ates I, Arikan MF, Erdogan K, Kaplan M, Yuksel M, Topcuoglu C, et al. Factors associated with increased irisin levels in the type 1 diabetes mellitus. Endocr Regul. 2017; 51:1–7. PMID: 28222023.

24. Huh JH, Ahn SV, Choi JH, Koh SB, Chung CH. High serum irisin level as an independent predictor of diabetes mellitus: a longitudinal population-based study. Medicine (Baltimore). 2016; 95:e3742. PMID: 27281072.
25. Tabak O, Simsek G, Erdenen F, Sozer V, Hasoglu T, Gelisgen R, et al. The relationship between circulating irisin, retinol binding protein-4, adiponectin and inflammatory mediators in patients with metabolic syndrome. Arch Endocrinol Metab. 2017; 61:515–523. PMID: 28977161.

26. Baran A, Mysliwiec H, Kiluk P, Swiderska M, Flisiak I. Serum irisin levels in patients with psoriasis. J Dermatolog Treat. 2017; 28:304–308. PMID: 27786588.

27. Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017; 13:324–337. PMID: 28211512.

28. Ebert T, Kralisch S, Wurst U, Scholz M, Stumvoll M, Kovacs P, et al. Association of metabolic parameters and rs726344 in FNDC5 with serum irisin concentrations. Int J Obes (Lond). 2016; 40:260–265. PMID: 26285604.

29. Mazur-Bialy AI, Bilski J, Pochec E, Brzozowski T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J Physiol Pharmacol. 2017; 68:243–251. PMID: 28614774.
30. Hurlimann J, Thorbecke GJ, Hochwald GM. The liver as the site of C-reactive protein formation. J Exp Med. 1966; 123:365–378. PMID: 4379352.

31. Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992; 4:361–368. PMID: 1420997.

32. Chi M, Tridandapani S, Zhong W, Coggeshall KM, Mortensen RF. C-reactive protein induces signaling through Fc gamma RIIa on HL-60 granulocytes. J Immunol. 2002; 168:1413–1418. PMID: 11801683.
33. Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993; 178:1629–1636. PMID: 7693853.

34. Chen N, Li Q, Liu J, Jia S. Irisin, an exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev. 2016; 32:51–59. PMID: 25952527.

35. Biniaminov N, Bandt S, Roth A, Haertel S, Neumann R, Bub A. Irisin, physical activity and fitness status in healthy humans: no association under resting conditions in a cross-sectional study. PLoS One. 2018; 13:e0189254. PMID: 29381744.

36. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013; 5:77–94. PMID: 23641259.

37. Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985). 2007; 103:1093–1098. PMID: 17347387.

38. Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998; 508(Pt 3):949–953. PMID: 9518745.

39. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999; 19:972–978. PMID: 10195925.
40. Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes Relat Metab Disord. 2002; 26:789–796. PMID: 12037649.

41. MacKenzie KE, Wiltshire EJ, Pena AS, Gent R, Hirte C, Piotto L, et al. Hs-CRP is associated with weight, BMI, and female sex but not with endothelial function in children with type 1 diabetes. Pediatr Diabetes. 2009; 10:44–51. PMID: 18798827.

42. Anastasilakis AD, Polyzos SA, Saridakis ZG, Kynigopoulos G, Skouvaklidou EC, Molyvas D, et al. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J Clin Endocrinol Metab. 2014; 99:3247–3255. PMID: 24915120.

43. Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008; 63:879–884. PMID: 18772478.

SUPPLEMENTARY MATERIALS
Supplemental Fig. S1
Forest plots of subgroup analyses based on (A) the disease status of the participants, (B) body mass index (BMI) of the participants. (C) Quality of the studies, (D) study design (cross-sectional vs. case-control vs. cohort). (E) Sex ratio (men vs. women), and (F) sample size. ES, effect size; CI, confidence interval; NOS, Newcastle-Ottawa Scale.
Supplemental Fig. S2
(A) Plot of the trim-and-fill analysis. (B) Plot of the sensitivity analysis results for the meta-analyses across all studies. CI, confidence interval.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download