Abstract
Whether or not Graves' hyperthyroidism can be really cured, depends on the definition of “cure.” If eradication of thyroid hormone excess suffices for the label “cure,” then all patients can be cured because total thyroidectomy or high doses of 131I will abolish hyperthyroidism albeit at the expense of creating another disease (hypothyroidism) requiring lifelong medication with levothyroxine. I would not call this a “cure,” which I would like to define as a state with stable thyroid stimulating hormone (TSH), free thyroxine, and triiodothyronine serum concentrations in the normal range in the absence of any thyroid medication. Surgery and radioiodine are unlikely to result in so-defined cures, as their preferable aim as stated in guidelines is to cause permanent hypothyroidism. Discontinuation of antithyroid drugs is followed by 50% recurrences within 4 years; before starting therapy the risk of recurrences can be estimated with the Graves' Recurrent Events After Therapy (GREAT) score. At 20-year follow-up about 62% had developed recurrent hyperthyroidism, 8% had subclinical hypothyroidism, and 3% overt hypothyroidism related to TSH receptor blocking antibodies and thyroid peroxidase antibodies. Only 27% was in remission, and might be considered cured. If the definition of “cure” would also include the disappearance of thyroid antibodies in serum, the proportion of cured patients would become even lower.
Graves' disease, can it be cured? A most relevant question for patients diagnosed with Graves' hyperthyroidism, which occurs in a substantial number of subjects as the prevalence in the general population is in the order of 1% to 1.5% [1]. The incidence is 20 to 30 cases per year per 100,000 persons [23].
Approximately 3% of women and 0.5% of men develop Graves' disease during their lifetime [2]. Graves' hyperthyroidism is the most common phenotype of Graves' disease; the other phenotypes Graves' orbitopathy (GO) and Graves' dermopathy (local myedema) are relatively rare and not taken into consideration in the following discussion on cure.
The natural history of Graves' hyperthyroidism is not well known. The main reason for this deficiency in our knowledge is that nowadays all patients with Graves' hyperthyroidism are treated in order to restore euthyroidism. Effective therapy with antithyroid drugs (ATDs) or radioactive iodine (RAI) has been available since the end of the Second World War, but even in the first half of the twentieth century patients could be successfully treated with thyroidectomy (Tx). Based on the older literature, attempts have been made to describe the natural history of Graves' hyperthyroidism [4]. Most patients (estimated 60% to 70%) follow an undulating course with alternating hyperthyroid and euthyroid episodes (Fig. 1, blue line). A minority (about 30% to 40%) experiences just one hyperthyroid episode (Fig. 1, green line). In some patients (not more than 10%) hyperthyroidism never remits and it is thought that in this group with perpetual hyperthyroidism the disease can be fatal if left untreated (Fig. 1, red line).
We can influence the course of Graves' hyperthyroidism by Tx, RAI, or ATD. Do these interventions really change the natural course of Graves' hyperthyroidism? Is the disease cured after a successful intervention?
If surgery is chosen for Graves' hyperthyroidism, current guidelines recommend total thyroidectomy (TTx) as the procedure of choice rather than subtotal thyroidectomy (STTx) [56]. TTx has a nearly 0% risk of recurrence, whereas STTx may have an 8% chance of persistence or recurrence of hyperthyroidism at 5 years [789]. Serum concentrations of thyroid-stimulating immunoglobulins (TSIs) rapidly decrease during the first 9 months after TTx but are still detected in 18% 3 years later [10]. One may conclude that TTx is capable to definitively cure the hyperthyroid state, but at the expense of creating another disease—hypothyroidism.
The above does not exclude the possibility to maintain a long-term remission of Graves' hyperthyroidism after STTx. A recent study enrolled 415 consecutive patients with Graves' hyperthyroidism who underwent bilateral STTx (n=385) or the Dunhill procedure (hemithyroidectomy+subtotal resection) (n=57) [11]. Median postoperative follow-up was 6 years. Persistent or recurrent hyperthyroidism occurred in 29%. Thyroid remnant weight was on average 5.1 g, and appeared to be an independent risk factor for persistent or recurrent hyperthyroidism (hazard ratio, 1.32). Hypothyroidism developed in over 50% of patients. Only 19% of patients remained euthyroid, and the rate did not increase significantly as thyroid remnant weight increased. This last finding does not support a recommendation done in the past to adjust the remnant size to the thyroid peroxidase or microsomal antibody (TPO-Ab) concentration in order to achieve the greatest likelihood of a postoperative euthyroid state: remnant size should be about 3 cm3 at low TPO-Ab concentrations but around 5 cm3 in case of high TPO-Ab levels [12]. It follows that STTx with the intent to maintain a euthyroid state, is not an optimal surgical strategy because the persistence or recurrence rate of Graves' hyperthyroidism is high and the euthyroid rate is low.
American Thyroid Association (ATA) guidelines stipulate the goal of RAI therapy (like that of surgery) in Graves' disease is to control hyperthyroidism by rendering the patient hypothyroid [5]. RAI is very effective provided a sufficient radiation dose is delivered in the thyroid. This can be done equally well by a fixed activity of 131I or by a calculated activity based on thyroid size and thyroid 131I uptake [5]. Balancing rapid relief of hyperthyroidism and postponing hypothyroidism appears to be an elusive goal. No dose calculation can secure long-term euthyroidism [6]. Many centers therefore have given up meticulous dose calculation and use fixed doses of 131I (e.g., 185, 370, or 555 MBq).
A recent study evaluating the use of an ablative RAI dose in 576 Graves' patients reports that 1 year after the first fixed 131I dose of 400 MBq, 17% was still hyperthyroid, 77% hypothyroid, and 6% euthyroid [13]. At 80-month follow-up, 81 patients had received a second dose and eight patients a third dose; 3.2% was still hyperthyroid (but controlled by ATD or Tx), 86.4% hypothyroid, 3.3% euthyroid, and 6.4% had died; no data in 0.7%. Persistent thyrotoxicosis after the first RAI dose was associated with higher free thyroxine (FT4) at diagnosis, higher pre-treatment thyroid stimulating hormone (TSH) receptor antibodies, and post-radioiodine treatment with ATD. New GO after RAI developed in 7.3%. RAI-associated GO is most likely related to a steep increase in TSH receptor antibodies in the first 6 months after RAI, with a slow decrease thereafter: 3 years after RAI, TSI are still positive in 60% of patients [1014]. Worsening or development of GO occurs more often after RAI than after treatment with ATD (38% vs. 19%; relative risk, 1.94; 95% confidence interval, 1.4 to 2.7) as evident from two randomized clinical trials (RCTs) [15]. Hypothyroidism developed in 95% after RAI, but euthyroidism was not achieved by any participant [15]. One can conclude that elimination of Graves' hyperthyroidism can be reached by RAI (although often a second or sometimes a third dose of 131I is required), but at the expense of a substantial risk of worsening or developing GO and creating a new disease—hypothyroidism. To replace one disease (hyperthyroidism) by another disease (hypothyroidism) and call that a “cure” of the original disease, seems odd. I would call a disease to be cured if, due to the spontaneous course of the disease or due to specific interventions, after a certain period of time nothing can be found any longer—be it clinical signs and symptoms or biochemical and radiological changes—attributable to that disease. In this context it is interesting to note the definition of remission in Graves' hyperthyroidism. In most studies remission is defined as normal serum TSH, FT4, and triiodothyronine (T3) serum concentrations lasting for 1 year without any treatment. But in a recent Australian study, remission was defined as euthyroidism or hypothyroidism after 12 months, and it was reported that 79% of patients achieved “remission” with a single dose of 131I [16].
RAI therapy might be considered problematic because of the need for lifelong levothyroxine replacement if the goal is to render the patient hypothyroid. A personalized dosimetric approach delayed the long-term onset of hypothyroidism in 26% of patients by using much lower administered activities than currently recommended [17]. An interesting RCT in China evaluated the feasibility of 131I therapy aiming at restoration of euthyroidism without the development of hypothyroidism [18]; realization of this goal would come closer to a “cure” of Graves' hyperthyroidism. Eligible patients were randomized into five groups of about 100 patients each. Patients received a range of varying 131I dosages depending on (1) group allocation: 0.37, 1.11, 1.85, 2.56, and 3.33 MBq/g thyroid tissue was given in group 1, 2, 3, 4, and 5 respectively; (2) clinical score: the score evaluated six items (each scored as 0, 1, or 2) about thyroid gland consistency, duration of disease, previous ATD treatment, severity of disease, complications, and age; the score ranges from 0 to 12, and 0.37 MBq/g was added for every 2 scores. Thus e.g., in group 3 the basic activity unit was 1.85 MBq/g and the therapeutic 131I activity remained 1.85 MBq/g at clinical score of 0, but increased to 4.07 MBq/g at a clinical score of 12 (1.85 MBq+6×0.37 MBq). In doing so one could compare the effect of various 131I dosages between homogeneous groups as patient characteristics did not differ between the five groups. The optimal outcome (highest proportion of euthyroidism and lowest proportion of hypothyroidism) was obtained in group 3 (average administered activity 261±162 MBq): at 12 year, 72% maintained euthyroidism, 6% remained hyperthyroid, and 22% became hypothyroid. Over the 12-year period, the total recurrence rate was 13.6% [17]. Whether this scheme is preferable to the intentional induction of permanent hypothyroidism recommended by ATA guidelines, remains doubtful.
The third option in the management of Graves' hyperthyroidism is ATDs. ATD have always been the treatment of choice for uncomplicated cases in Europe and Japan, but RAI was the favourite treatment modality in the USA. A 2011 survey reported ATD were preferred by 86% in Europe and 40% in North America, whereas RAI was preferred by 13% in Europe and 59% in the USA [19]. This has changed dramatically in the USA in the last decade, and now ATD appear the most common treatment in the USA used in 58% of patients followed by RAI in 35% [20]. The reason for this shift away from RAI towards ATD might be the realization that RAI is associated with a definite risk for developing or worsening of thyroid eye disease [1415]. Could it be that this shift towards ATD is also related to a greater chance of “cure” of Graves' hyperthyroidism after ATD than after RAI?
To know before starting ATD the likelihood of remission after completing a course of ATD, would be very relevant for selecting the most appropriate treatment modality in a particular patient. If the chance of remission is low, Tx or RAI might be a better option for that patient. Factors associated with a low remission rate as suggested in many but not all studies, are male sex, young age (<40 years), smoking, severe hyperthyroidism, high concentrations of thyrotropin binding inhibitory immunoglobulins (TBIIs), large goiter size, and the presence of GO [2122]. However, the predictive value of each of these risk factors is too low for accurate assessment of the remission chance before starting ATD in the individual patient. A recent prospective study was able to construct a predictive score by combining a number of independent risk factors. This so-called Graves' Recurrent Events After Therapy (GREAT) score provides a reasonable prediction of recurrent Graves' hyperthyroidism after an 18-months course of ATD [23]. The GREAT score takes into account four baseline characteristics, which are already assessed routinely in the work-up of every patient with Graves' hyperthyroidism: age, FT4, TBII, and goiter size (Table 1) [56]. Patients in whom the GREAT score falls in class I, have a rather high chance on remission (84%), and ATD would be a reasonable option. In contrast, chance on remission is rather low in GREAT score class III (32%), and Tx or RAI might be preferred. Chance on remission (56%) or recurrence (44%) is about even in patients falling in GREAT score class II; in these patients adding the results of genotyping (human leukocyte antigen subtypes DQB1-02, DQA1-05, DRB1-03, and PTPN22 C/T) generates the GREAT+ score which has a greater predictive value and may change management in 38% of patients [23]. The predictive value of the GREAT score has subsequently been validated by two other independent studies [2425]. Its accuracy might possibly be enhanced by replacing TBII by an assay which more specifically measures TSH receptor stimulating antibodies [2627]. It is also foreseen that the predictive value of the GREAT+ score (which incorporates genotyping) could be enhanced by adding more genotypes related to Graves' disease (like CTLA-4 G/G and TSHR) [2829].
A single course of ATD induces remission of Graves' hyperthyroidism in about 50% of cases [32130]. Remission rates vary greatly, however, from 30% up to 70% in individual studies. Remission rates are only weakly related to the duration of ATD treatment: 12 to 18 months seems optimal, with slightly higher recurrence rates after 6 months and no apparent additional benefit by extending therapy beyond 18 months [21]. Remission rates do not differ between the titration method (in which the ATD dose is adjusted according to laboratory results) or the block-and-replace method (in which the relatively high ATD starting dose is maintained and levothyroxine is added when euthyroidism has been reached) [3132]. Excessive iodine intake does not influence remission rates in iodine-replete areas [33]. The mechanisms by which ATD induce a remission, are not completely understood. It might be related to direct effects of ATD on intrathyroidal T-cells [34], but indirect effects on the immune system by restoration of the euthyroid state might play a role as well [35].
Attempts to enhance remission rates so far failed. Administration of levothyroxine after discontinuation of ATD seemed to increase remission rate [36], but subsequent studies could not confirm the initially promising results and this particular treatment modality is not used any longer [3738]. Likewise, adding selenium to ATD did increase remission rate in a pilot study [39], but not in a subsequent placebo-controlled RCT [40].
It is thought unlikely that patients with recurrent Graves' hyperthyroidism would go into remission after a second course of ATD, and guidelines therefore recommend definitive treatment by RAI or Tx in case of recurrences. But the last American and European guidelines mention that a second course of ATD might be considered [56]. In one study from China a second course of ATD lasting for 15 to 20 months resulted in 76% remissions at a follow-up of 4 years [41]. A Korean study demonstrated similar remission and relapse rates between the first and second course of ATD with 10-year remission rates of 34% and 25% respectively, whereas 10-year remission rates were progressively lower after the third and fourth course of ATD (17% and 13%, respectively) [42].
If TBII is negative at the end of 12 to 18 months of ATD therapy, it is reasonable to discontinue ATD as chance on remission is relatively high. Remission chance is relatively low in the presence of high TSH receptor antibody levels at the end of ATD therapy, a condition sometimes referred to as persistent hyperthyroidism although serum thyroid hormones are normal [6]. It reflects the notion that Graves' hyperthyroidism is not really cured as long as TSH receptor antibodies are present, and I quite agree with this line of thinking. Current guidelines acknowledge the feasibility of continuing ATD for a further 12 months if TBII is still high, or to proceed with long-term use of ATD (usually a low dose of methimazole) [56]. This strategy has been employed specifically in patients with co-existent GO. In a Dutch study ATD were discontinued after a median treatment duration of 3.5 years (range, 2 to 11): during a median follow-up period of 5 years (range, 1 to 14) recurrent Graves' hyperthyroidism occurred in 37% (easily managed with 131I), without a relapse of GO [43]. Long-term treatment with a low-dose of methimazole (5 mg/day, n=101) or propylthiouracil (200 mg/day, n=7) for a median duration of 6.7 years in patients with co-existent severe GO, is reported from Denmark: 90% maintained euthyroidism during treatment, and the remaining 10% experienced relapse of hyperthyroidism either spontaneously or after ATD dose reduction [44]. TSH receptor antibodies gradually disappeared from serum in most patients. The only serious side effect of ATD was vasculitis occurring after 6 years of PTU in a 48-year-old woman [44]. A meta-analysis of six studies in which Graves' hyperthyroidism had been treated with ATD for ≥2 years, finds a remission rate of 57%, and a complication rate of 19% (major complications only in 1.5%) [45]. Smoking had a significant lowering effect on remission rate. In a study from Iran patients who had failed to achieve remission on ATD, were randomized into either RAI or further ATD therapy: 10-year outcomes were almost similar with regard to expenses, but hypothyroid episodes were more frequent after RAI than during ATD [46]. A nonrandomized study from Brazil also compared RAI with prolonged low-dose methimazole treatment in patients with recurrent Graves' hyperthyroidism after a course of ATD: RAI was associated with worsening of GO, more weight gain, and more often hypothyroidism at a follow-up of 5 years [47].
The long-term outcome of stable remissions is less well known. We have to rely on studies performed in the 1970s and 1980s, when sensitive TSH assays were not yet available and a single TSH measurement could not discriminate between suppressed and normal TSH values. Consequently the TSH response to TRH was often used. Combining the results of three studies, 170 patients were assembled who were in stable remission for more than 10 years [484950]. Euthyroidism was observed in 60%, subclinical hyperthyroidism in 16%, subclinical hypothyroidism in 18%, and hypothyroidism in 6% (Table 2).
In one of these studies thyroid histology was available in eight euthyroid patients in longstanding remission: six of them had chronic lymphocytic thyroiditis like in Hashimoto thyroiditis, and none showed diffuse epithelial hyperplasia like in Graves' disease [50]. In the same study, microsomal antibodies (TPO-Ab) had been measured in 22 patients at the time of discontinuation ATD and 10 years later; TPO-Ab were positive in 59% and 91% respectively [50]. Another paper investigated 26 patients who developed hypothyroidism 0.5 to 10 years after discontinuation of ATD therapy [51]. Histology showed chronic lymphocytic thyroiditis in eight patients (fibrous variant in three of them) and partial epithelial hyperplasia in one patient. Microsomal antibodies (TPO-Ab) were present in all, TSH blocking antibodies in 33% and thyroid stimulating antibodies in 66%. TSH blocking antibodies may account for approximately 33% and chronic lymphocytic thyroiditis for 66% of these hypothyroid cases [51]. The presence of TPO-Ab is apparently related to the late development of hypothyroidism in Graves' disease, which comes as no surprise as TPO-Ab is a well-known risk factor for the development of auto-immune hypothyroidism [52]. It is of interest that the presence of TPO-Ab on the other hand seems to protect against recurrent Graves' hyperthyroidism. One hundred and seventeen patients with Graves' hyperthyroidism were treated with ATD for 2 years and then followed for on average 2.5 years; they were divided in group 1 (no thyroglobulin antibody [Tg-Ab] and no TPO-Ab), group 2 (no Tg-Ab but positive TPO-Ab), and group 3 (positive Tg-Ab and positive TPO-Ab) before and during ATD therapy. Relapse rates in the three groups were 39%, 27%, and 11% respectively [53]. A more recent study likewise reports that baseline TPO-Ab is inversely associated with relapse rates in a dose-dependent manner [54]. The data suggest patients with TPO-Ab are least likely to relapse, but more likely to proceed to hypothyroidism. However, other studies did not find TPO-Ab measurements useful for prediction of remissions or recurrences [5556].
The perplexing conclusion of very long-term follow-up studies in Graves' patients in stable remission is that 40% have still abnormal thyroid function, related to the persistence of thyroid antibodies (Table 2). In 16% there exists subclinical hyperthyroidism, most likely due to the persistence of TSH receptor stimulating antibodies. In 24% there exists subclinical or overt hypothyroidism, related to either TSH receptor blocking antibodies or TPO-Abs. Interestingly, many patients in this group also have persistent TSH receptor stimulating antibodies. It illustrates again how the interplay between destructive/inhibitory and stimulatory immunological effector mechanisms determines the outcome of thyroid function in autoimmune diseases [57]. The co-existence of TSH receptor stimulating and blocking antibodies is of particular pathophysiological interest. Remissions of Graves' hyperthyroidism are caused by a decrease of TSH rereceptor stimulating antibodies, but can an increase in TSH receptor blocking antibodies contribute to remissions? Alternating hyperthyroidism and hypothyroidism in Graves' disease has indeed been linked to switches between TSH receptor stimulating and blocking antibodies [58]. Can hypothyroidism induced by TSH receptor blocking antibodies still be called Graves' disease? Have hypothyroid patients with both TSH receptor blocking antibodies and TPO-Abs two simultaneous diseases, Graves' disease and Hashimoto thyroiditis? Or do they belong to one and the same disease entity, namely autoimmune thyroid disease? Apart from these semantical issues, it is clear that Graves' hyperthyroidism cannot be called really cured in the presence of still abnormal thyroid function.
Whether or not Graves' hyperthyroidism can be cured, depends on the definition of ‘cure.’ If cure is defined as just disappearance of thyroid hormone excess, then cure is possible in almost all cases by either Tx, RAI, or ATD. Cure defined as ‘restitutio ad integrum’ implies maintenance of the euthyroid state like it was before the illness, that is without any medication and without thyroid antibodies in the circulation. Guidelines state the aim of Tx or RAI in Graves' hyperthyroidism should be permanent hypothyroidism, which requires lifelong levothyroxine medication for maintaining euthyroidism. Creating another disease in order to treat the original disease, is no cure. ATDs leave open the possibility of cure, defined as maintenance of the euthyroid state (normal TSH, FT4, and T3) without the use of any medication.
The average remission rate after a course of ATD is about 50% [21]. Most recurrences occur within 4 years after discontinuation of ATD [3]. Although prognosis is excellent after 4 years without relapse [30], late recurrences do occur and only one in three patients experiences permanent remission [21]. Remission rate after 10 years is in the order of 30% to 40%, and hypothyroidism has developed in 10% to 15% 15 years after ATD [59]. Taken into account the above reviewed literature, permanent cure of Graves' hyperthyroidism is possible albeit at a low rate of about 27% (Fig. 2). The cure rate would be even lower if cure also supposes the absence of TSH receptor antibodies.
Figures and Tables
Fig. 1
Hypothetical curves reflecting the natural history of Graves' hyperthyroidism. A minority of patients (green line) have a single episode of hyperthyroidism. The majority (blue line) has a prolonged course following a relapsing and remitting course over many years. In some patients (red line) the disease never remits but continues to express herself clinically. In the long-term the natural course along the green and blue curves could evolve towards spontaneous development of hypothyroidism.
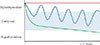
Fig. 2
Chance of remission of Graves' hyperthyroidism after a course of antithyroid drugs. ATD, antithyroid drug.

Table 1
A Predictive Score (Called the GREAT Score) for the Outcome of Therapy with Antithyroid Drugs in Graves' Hyperthyroidism Based on Four Baseline Characteristics [23]
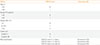
GREAT, Graves' Recurrent Events After Therapy; FT4, free thyroxine; TBII, thyrotropin binding inhibitory immunoglobulin.
aWorld Health Organization grade 0=thyroid not or distinctly palpable; grade I=thyroid easily palpable and visible with head in normal or raised position; grade II=thyroid easily visible with head in normal position; grade III=goitre visible at a distance.
References
2. Nystrom HF, Jansson S, Berg G. Incidence rate and clinical features of hyperthyroidism in a long-term iodine sufficient area of Sweden (Gothenburg) 2003-2005. Clin Endocrinol (Oxf). 2013; 78:768–776.

3. Hussain YS, Hookham JC, Allahabadia A, Balasubramanian SP. Epidemiology, management and outcomes of Graves' disease-real life data. Endocrine. 2017; 56:568–578.



4. Wass JAH, Stewart PM. Chapter 3.3.6, Antithyroid drug treatment for thyrotoxicosis. Oxford textbook of endocrinology and diabetes. 2nd ed. Oxford: Oxford University Pres;2011. p. 476–480.
5. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016; 26:1343–1421.


6. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves' hyperthyroidism. Eur Thyroid J. 2018; 7:167–186.



7. Guo Z, Yu P, Liu Z, Si Y, Jin M. Total thyroidectomy vs bilateral subtotal thyroidectomy in patients with Graves' diseases: a meta-analysis of randomized clinical trials. Clin Endocrinol (Oxf). 2013; 79:739–746.

8. Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E. What is the best definitive treatment for Graves' disease? A systematic review of the existing literature. Ann Surg Oncol. 2013; 20:660–667.

9. Liu ZW, Masterson L, Fish B, Jani P, Chatterjee K. Thyroid surgery for Graves' disease and Graves' ophthalmopathy. Cochrane Database Syst Rev. 2015; (11):CD010576.

10. Kautbally S, Alexopoulou O, Daumerie C, Jamar F, Mourad M, Maiter D. Greater efficacy of total thyroidectomy versus radioiodine therapy on hyperthyroidism and thyroid-stimulating immunoglobulin levels in patients with Graves' disease previously treated with antithyroid drugs. Eur Thyroid J. 2012; 1:122–128.



11. Lin YS, Lin JD, Hsu CC, Yu MC. The long-term outcomes of thyroid function after subtotal thyroidectomy for Graves' hyperthyroidism. J Surg Res. 2017; 220:112–118.


12. Huang CS, Wang M, Shun CT, Liaw KY. Factors affecting thyroid function after thyroidectomy for Graves' disease. J Formos Med Assoc. 1995; 94:423–427.

13. Aung ET, Zammitt NN, Dover AR, Strachan MWJ, Seckl JR, Gibb FW. Predicting outcomes and complications following radioiodine therapy in Graves' thyrotoxicosis. Clin Endocrinol (Oxf). 2019; 90:192–199.


14. Laurberg P, Wallin G, Tallstedt L, Abraham-Nordling M, Lundell G, Torring O. TSH-receptor autoimmunity in Graves' disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008; 158:69–75.


15. Ma C, Xie J, Wang H, Li J, Chen S. Radioiodine therapy versus antithyroid medications for Graves’ disease. Cochrane Database Syst Rev. 2016; 2:CD010094.

16. Fanning E, Inder WJ, Mackenzie E. Radioiodine treatment for Graves' disease: a 10-year Australian cohort study. BMC Endocr Disord. 2018; 18:94.



17. Hyer SL, Pratt B, Gray M, Chittenden S, Du Y, Harmer CL, et al. Dosimetry-based treatment for Graves' disease. Nucl Med Commun. 2018; 39:486–492.



18. Chen DY, Schneider PF, Zhang XS, He ZM, Jing J, Chen TH. Striving for euthyroidism in radioiodine therapy of Graves' disease: a 12-year prospective, randomized, open-label blinded end point study. Thyroid. 2011; 21:647–654.


19. Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves' disease. J Clin Endocrinol Metab. 2012; 97:4549–4558.


20. Brito JP, Schilz S, Singh Ospina N, Rodriguez-Gutierrez R, Maraka S, Sangaralingham LR, et al. Antithyroid drugs-the most common treatment for Graves' disease in the United States: a nationwide population-based study. Thyroid. 2016; 26:1144–1145.


21. Burch HB, Cooper DS. Anniversary review: antithyroid drug therapy: 70 years later. Eur J Endocrinol. 2018; 179:R261–R274.
22. Struja T, Fehlberg H, Kutz A, Guebelin L, Degen C, Mueller B, et al. Can we predict relapse in Graves' disease? Results from a systematic review and meta-analysis. Eur J Endocrinol. 2017; 176:87–97.


23. Vos XG, Endert E, Zwinderman AH, Tijssen JG, Wiersinga WM. Predicting the risk of recurrence before the start of antithyroid drug therapy in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab. 2016; 101:1381–1389.


24. Struja T, Kaeslin M, Boesiger F, Jutzi R, Imahorn N, Kutz A, et al. External validation of the GREAT score to predict relapse risk in Graves' disease: results from a multicenter, retrospective study with 741 patients. Eur J Endocrinol. 2017; 176:413–419.


25. Masiello E, Veronesi G, Gallo D, Premoli P, Bianconi E, Rosetti S, et al. Antithyroid drug treatment for Graves' disease: baseline predictive models of relapse after treatment for a patient-tailored management. J Endocrinol Invest. 2018; 41:1425–1432.


26. Giuliani C, Cerrone D, Harii N, Thornton M, Kohn LD, Dagia NM, et al. A TSHR-LH/CGR chimera that measures functional thyroid-stimulating autoantibodies (TSAb) can predict remission or recurrence in Graves' patients undergoing antithyroid drug (ATD) treatment. J Clin Endocrinol Metab. 2012; 97:E1080–E1087.

27. Kwon H, Kim WG, Jang EK, Kim M, Park S, Jeon MJ, et al. Usefulness of measuring thyroid stimulating antibody at the time of antithyroid drug withdrawal for predicting relapse of Graves disease. Endocrinol Metab (Seoul). 2016; 31:300–310.



28. Eliana F, Suwondo P, Asmarinah A, Harahap A, Djauzi S, Prihartono J, et al. The role of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) gene, thyroid stimulating hormone receptor (TSHR) gene and regulatory t-cells as risk factors for relapse in patients with Graves disease. Acta Med Indones. 2017; 49:195–204.

29. Garcia-Mayor RV, Alvarez-Vazquez P, Fluiters E, Valverde D, Andrade A. Long-term remission following antithyroid drug withdrawal in patients with Graves' hyperthyroidism: parameters with prognostic value. Endocrine. 2019; 63:316–322.


30. Mohlin E, Filipsson Nystrom H, Eliasson M. Long-term prognosis after medical treatment of Graves' disease in a northern Swedish population 2000-2010. Eur J Endocrinol. 2014; 170:419–427.


31. Abraham P, Avenell A, Park CM, Watson WA, Bevan JS. A systematic review of drug therapy for Graves' hyperthyroidism. Eur J Endocrinol. 2005; 153:489–498.


32. Razvi S, Vaidya B, Perros P, Pearce SH. What is the evidence behind the evidence-base? The premature death of block-replace antithyroid drug regimens for Graves' disease. Eur J Endocrinol. 2006; 154:783–786.


33. Park SM, Cho YY, Joung JY, Sohn SY, Kim SW, Chung JH. Excessive iodine intake does not increase the recurrence rate of Graves' disease after withdrawal of the antithyroid drug in an iodine-replete area. Eur Thyroid J. 2015; 4:36–42.



34. Volpe R. The immunomodulatory effects of anti-thyroid drugs are mediated via actions on thyroid cells, affecting thyrocyte-immunocyte signalling: a review. Curr Pharm Des. 2001; 7:451–460.


35. Laurberg P. Remission of Graves’ disease during anti-thyroid drug therapy. Time to reconsider the mechanism? Eur J Endocrinol. 2006; 155:783–786.


36. Hashizume K, Ichikawa K, Sakurai A, Suzuki S, Takeda T, Kobayashi M, et al. Administration of thyroxine in treated Graves' disease. Effects on the level of antibodies to thyroid-stimulating hormone receptors and on the risk of recurrence of hyperthyroidism. N Engl J Med. 1991; 324:947–953.

37. Tamai H, Hayaki I, Kawai K, Komaki G, Matsubayashi S, Kuma K, et al. Lack of effect of thyroxine administration on elevated thyroid stimulating hormone receptor antibody levels in treated Graves' disease patients. J Clin Endocrinol Metab. 1995; 80:1481–1484.


38. Mori T, Sugawa H, Kosugi S, Ueda M, Hai N, Matsuda A. Recent trends in the management of Graves' hyperthyroidism in Japan: opinion survey results, especially on the combination therapy of antithyroid drug and thyroid hormone. Endocr J. 1997; 44:509–517.


39. Wang L, Wang B, Chen SR, Hou X, Wang XF, Zhao SH, et al. Effect of selenium supplementation on recurrent hyperthyroidism caused by Graves' disease: a prospective pilot study. Horm Metab Res. 2016; 48:559–564.


40. Kahaly GJ, Riedl M, Konig J, Diana T, Schomburg L. Double-blind, placebo-controlled, randomized trial of selenium in Graves hyperthyroidism. J Clin Endocrinol Metab. 2017; 102:4333–4341.


41. Liu X, Qiang W, Liu X, Liu L, Liu S, Gao A, et al. A second course of antithyroid drug therapy for recurrent Graves' disease: an experience in endocrine practice. Eur J Endocrinol. 2015; 172:321–326.



42. Kim YA, Cho SW, Choi HS, Moon S, Moon JH, Kim KW, et al. The second antithyroid drug treatment is effective in relapsed Graves' disease patients: a median 11-year follow-up study. Thyroid. 2017; 27:491–496.


43. Elbers L, Mourits M, Wiersinga W. Outcome of very long-term treatment with antithyroid drugs in Graves' hyperthyroidism associated with Graves' orbitopathy. Thyroid. 2011; 21:279–283.


44. Laurberg P, Berman DC, Andersen S, Bulow Pedersen I. Sustained control of Graves' hyperthyroidism during long-term low-dose antithyroid drug therapy of patients with severe Graves' orbitopathy. Thyroid. 2011; 21:951–956.


45. Azizi F, Malboosbaf R. Long-term antithyroid drug treatment: a systematic review and meta-analysis. Thyroid. 2017; 27:1223–1231.


46. Azizi F, Ataie L, Hedayati M, Mehrabi Y, Sheikholeslami F. Effect of long-term continuous methimazole treatment of hyperthyroidism: comparison with radioiodine. Eur J Endocrinol. 2005; 152:695–701.


47. Villagelin D, Romaldini JH, Santos RB, Milkos AB, Ward LS. Outcomes in relapsed Graves' disease patients following radioiodine or prolonged low dose of methimazole treatment. Thyroid. 2015; 25:1282–1290.


48. Irvine WJ, Gray RS, Toft AD, Seth J, Lidgard GP, Cameron EH. Spectrum of thyroid function in patient's remaining in remission after antithyroid drug therapy for thyrotoxicosis. Lancet. 1977; 2:179–181.


49. Wood LC, Ingbar SH. Hypothyroidism as a late sequela in patient with Graves’ disease treated with antithyroid agents. J Clin Invest. 1979; 64:1429–1436.


50. Hirota Y, Tamai H, Hayashi Y, Matsubayashi S, Matsuzuka F, Kuma K, et al. Thyroid function and histology in forty-five patients with hyperthyroid Graves' disease in clinical remission more than ten years after thionamide drug treatment. J Clin Endocrinol Metab. 1986; 62:165–169.


51. Tamai H, Kasagi K, Takaichi Y, Takamatsu J, Komaki G, Matsubayashi S, et al. Development of spontaneous hypothyroidism in patients with Graves' disease treated with antithyroidal drugs: clinical, immunological, and histological findings in 26 patients. J Clin Endocrinol Metab. 1989; 69:49–53.


52. Strieder TG, Tijssen JG, Wenzel BE, Endert E, Wiersinga WM. Prediction of progression to overt hypothyroidism or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Intern Med. 2008; 168:1657–1663.


53. Takaichi Y, Tamai H, Honda K, Nagai K, Kuma K, Nakagawa T. The significance of antithyroglobulin and antithyroidal microsomal antibodies in patients with hyperthyroidism due to Graves' disease treated with antithyroidal drugs. J Clin Endocrinol Metab. 1989; 68:1097–1100.


54. Stefanic M, Karner I. Thyroid peroxidase autoantibodies are associated with a lesser likelihood of late reversion to hyperthyroidism after successful non-ablative treatment of Graves' disease in Croatian patients. J Endocrinol Invest. 2014; 37:71–77.


55. Lin HD, Tai FT, Chen HD, Lee SP, Chang FY, Wang GG, et al. Change of circulating thyroid autoantibody titers in Graves' hyperthyroidism after antithyroid drugs therapy. Zhonghua Yi Xue Za Zhi (Taipei). 1991; 47:86–90.

56. Schott M, Eckstein A, Willenberg HS, Nguyen TB, Morgenthaler NG, Scherbaum WA. Improved prediction of relapse of Graves' thyrotoxicosis by combined determination of TSH receptor and thyroperoxidase antibodies. Horm Metab Res. 2007; 39:56–61.


57. Gill RG, Harmon JT, McLaren NK. Chapter 18, Autoimmune thyroid diseases. Immunologically mediated endocrine diseases. Philadelphia: Lippincott Williams & Wilkins;2002. p. 373–396.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download