Abstract
Background
Iodine deficiency (ID) has become a concern not only among pregnant women, but in women of childbearing age as well. In fact, a recent report suggested that women with moderate to severe ID may experience a significantly longer time to conceive. This study aimed to investigate iodine status in Filipino women of childbearing age.
Methods
The iodine status of 6,194 Filipino women aged 15 to 45 years old was assessed through urinary iodine analysis. A casual spot urine sample was collected from women in households participating in the eighth National Nutrition Survey conducted by the Food and Nutrition Research Institute. The sample was analyzed using ammonium persulfate digestion followed by the Sandell-Kolthoff colorimetric reaction. A median urinary iodine concentration (UIC) of less than 100 µg/L was used to define ID.
Results
The median UIC was 123 µg/L, indicative of adequate iodine nutrition; however, 21.5% of participants had a UIC below 50 µg/L. The median UIC of women who lived in urban areas (142 µg/L), belonged to the middle to richest class (>124 µg/L), had reached a college education (136 µg/L), and used iodized salt (15 ppm and above; 148 to 179 µg/L) reflected adequate iodine nutrition. ID was found to have been eliminated in the regions of Central Luzon, Eastern Visayas, Calabarzon, Mimaropa, and the National Capital, while mild ID was identified in Western Visayas, Southern and Western Mindanao, and in the Autonomous Region in Muslim Mindanao.
The World Health Organization has asserted that iodine is one of the most critical micronutrients for maternal and child health. Severe iodine deficiency (ID) during pregnancy can cause both maternal and fetal hypothyroidism. Moreover, it is associated with adverse obstetric outcomes, including stillbirth, spontaneous abortion, and prematurity [1].
While it is essential for women to consume adequate levels of iodine during pregnancy, it is equally important that women of childbearing age consume sufficient amounts of iodine, especially those who are planning a pregnancy. According to recent studies, a better thyroid profile has been observed among pregnant women who had regular adequate iodine intake before they became pregnant than among women who began iodine supplementation upon becoming pregnant [234].
Because the first trimester is a critical period for sufficient thyroid hormone levels and most pregnancies are unplanned, it is important for women of childbearing age to consume an adequate amount of iodine, corresponding to the recommended iodine intake of 150 µg/day [5].
Since the beginning of the 21st century, iodine nutrition among Filipino pregnant and lactating women has been assessed based on urinary iodine concentration (UIC). National nutrition surveys have revealed that these physiologic groups did not have adequate iodine levels from 2003 to 2013 [6]. ID has become a concern not only among American pregnant women, but in women of childbearing age as well [7]. Reported UIC levels below the target value of 100 µg/L have been recorded in more than 30% of childbearing-age women in the United States [89]. A recent population-based cohort study suggested that women with moderate to severe ID may experience significantly longer time to conceive and diminished fecundity [10].
With the onset of the concept of nutrition in the first 1,000 days of life, the critical importance of the nutritional status of childbearing-age women has been underscored. This concept highlights that for women to be ready to become healthy mothers in the future, their micronutrient status should be secured. This paper assessed the iodine status of 15- to 45-year-old, non-pregnant, non-lactating Filipino women. Likewise, it compared the median UICs of the women in terms of place of residence, wealth quintile, levels of iodine in salt, and educational attainment.
The conduct of the study was approved by the Food and Nutrition Research Institute (FNRI) Institutional Ethics and Review Committee. It was carried out in accordance with the Declaration of Helsinki, guided by the Council for International Organization of Medical Sciences Ethical Guidelines for Biomedical Research involving human subjects [11] and the National Guidelines for Biomedical/Behavioral Research [12]. Written consent to participate in the National Nutrition Survey (NNS) was obtained from all participants prior to the interview, urine collection, and other measurements. Only those who signed the informed consent form were included in the study.
The Philippine's NNS is a cross-sectional survey conducted by the Department of Science and Technology-Food and Nutrition Research Institute (DOST-FNRI) every 5 years and covers all the 17 regions of the country. The NNS was designed to provide national and sub-national estimates of the nutritional status of the population [13]. A stratified multi-stage sampling design was used, beginning with the selection of primary sampling units, which were defined as a barangay or contiguous barangays with at least 500 households. This was followed by the selection of enumeration areas, which were defined as a contiguous area in a barangay or a barangay with 150 to 200 households. The final sampling unit was the household and all its members.
Mid-stream, casual samples of roughly 15 mL of urine were collected in a non-fasting state from 6,194 women of childbearing age (15 to 45 years old) in the participating households. These were kept frozen in the field and while in transport to the Biochemical Laboratory (BL) of DOST-FNRI. At the BL, the urine samples were stored in freezers below −20℃ until analysis.
Urinary iodine determination was conducted at the BL of the DOST-FNRI. The acid digestion method of Pino et al. [14] was used to determine UIC. For quality assurance, the BL participated in the Ensuring Quality of Urinary Iodine Procedures (EQUIP) program of the Centers for Disease Control and Prevention in Atlanta, GA, USA. For precision, several urine samples with a predetermined UIC were analyzed together with the survey samples. To evaluate the accuracy of the method, the laboratory used a certified reference material (Seronorm Trace Elements Urine, SERO, Oslo, Norway). The mean value for Seronorm Trace Elements Urine L-1 was 101 µg/L (certified mean, 105; range, 84 to 126), while the mean value for Seronorm Trace Elements Urine L-2 was 325 µg/L (certified mean, 297; range, 237 to 356).
Data on the socioeconomic status (SES) of the women were collected from all survey participants through face-to-face interviews. Similarly, approximately 100 g of salt from the household was obtained for iodine analysis using a WYD checker (Kejing, Tianjin, China). In brief, 1 g of a salt sample was mixed with a potassium iodide-starch solution and distilled water in a tube. The tube was placed in the cell holder of the WYD checker and the iodine concentration was instantaneously displayed on the instrument.
Descriptive statistics (medians and prevalence of deficiencies) were generated using Stata version 15.0 (StataCorp, College Station, TX, USA) and R version 3.5 (R Foundation for Statistical Computing, Vienna, Austria). The severity of ID in women of childbearing age based on median UIC was assessed using the epidemiological criteria set by the World Health Organization, United Nations Children's Fund, and International Council for the Control of Iodine Deficiency Disorders [1], as shown in Table 1. The distribution of UIC was compared using a design-based rank test [15].
Furthermore, elimination of ID was defined a median value of 100 µg/L—that is, 50% of the sample should be above 100 µg/L—with no more than 20% of the samples below 50 µg/L.
The eighth NNS was conducted in 17 regions of the Philippines, consisting of 80 provinces. A total of 6,194 women of childbearing age participated in the survey.
Table 2 shows the median UIC and the proportions of values less than 50 µg/L and values greater than or equal to 100 µg/L by region. The median UIC of 123 µg/L indicates that this population as a whole had optimal iodine nutrition.
ID was found to have been eliminated in the Central Luzon, Calabarzon, Mimaropa, Eastern Visayas, and the National Capital regions. In these regions, the median UIC was above 100 µg/L and the percentage of samples with less than 50 µg/L was below 20%. Furthermore, Fig. 1 illustrates the regional iodine status of Filipino women of childbearing age based on median UIC.
Table 3 presents the distribution of UIC by region. On average, roughly 10% of the population in these regions had severe ID (below 20 µg/L). Mild ID existed in four regions: the Autonomous Region in Muslim Mindanao, Western Mindanao, Southern Mindanao, and Western Visayas. In these regions, more than 20% of women had a UIC below 50 µg/L. This suggests that the Philippines has not yet eliminated ID among women of childbearing age, since some regions still had a median below 100 µg/L.
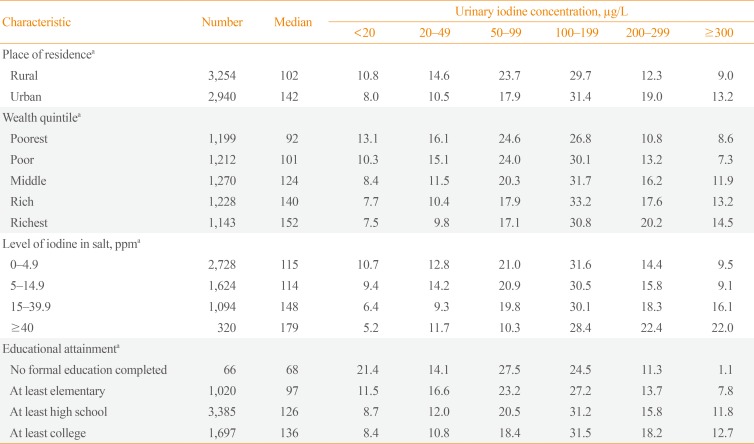
The median UIC was lower in women residing in rural areas (P<0.001) than in women living in urban areas (Table 4). Furthermore, the proportion of women in rural areas with a UIC below 50 µg/L was above 20% (25%).
In terms of wealth, the median UIC increased as the wealth quintile increased from the poorest to the richest group, as presented in Table 4 (P<0.001). The median UIC was 92 µg/L in the poorest quintile and 152 µg/L in the richest quintile. In addition, more than 20% of women had values below 50 µg/L in the poorest (29.2%) and poor (25.4%) groups.
The women were also grouped based on the level of iodine detected in the salt collected from their households. They were disaggregated according to iodine level as follows: 0 to 4.9 mg of iodine per kilogram of salt (parts per million, ppm), 5 to 14.9, 15 to 39.9, and ≥40 ppm. The median UIC among all these groups was more than 100 µg/L. However, 23.5% and 23.6% of women in the 0 to 4.9 and 5 to 14.9 ppm groups, respectively, had a UIC below 50 µg/L.
Table 4 also shows that the women who had not completed any formal education (n=66) had the worst median UIC (68 µg/L). This result shows a direct positive relationship between the median UIC and educational attainment.
It is known that an insufficient supply of thyroid hormone to the developing brain results in neurocognitive impairment [16171819]. This may correspond to the fetal development phase, which happens between the first and second trimesters of pregnancy. Therefore, the children of iodine-deficient mothers are at a higher risk of diminished cognition [20]. Thus, ensuring optimal and adequate iodine intake from the moment a woman plans to become pregnant is particularly important for preventing adverse effects in their offspring, because even slightly low maternal thyroid hormone levels during pregnancy can cause cognitive delays in their offspring [21].
In this study, the median UIC (123 µg/L) among Filipino women of childbearing age reflected adequate iodine intake. This level is similar to that of school-age children (SAC), the globally-accepted proxy of the iodine status of the entire population, who were assessed as having a median UIC of 168 µg/L in the same survey. However, the percentage of UIC below 50 µg/L in women was 21.5%, while the corresponding percentage in SAC was only 16.4% [22]. Some regions also had a median UIC below 100 µg/L. This means that ID among childbearing-age women in the Philippines has not yet been eliminated.
Globally, the iodine status in women of childbearing age varies. For instance, in non-pregnant, non-lactating women in Sierra Leone aged 15 to 49 years old, all subgroups had a median substantially above the threshold of 100 µg/L [23]. Similarly, Indonesian and Australian women were found to have sufficient iodine intake, based on median UIC values of 189.0 and 117 µg/L, respectively [2425]. In contrast, mild ID was observed among women of childbearing age in Samoa and New Zealand [2627].
Although a few reports have observed better iodine nutrition in coastal areas [2829], no specific geographic foci can be assumed to be indicative of low-prevalence areas. Living on the seacoast does not guarantee iodine sufficiency. In fact, other studies revealed that proximity to the coast had no impact on iodine status at all [3031]. In this study, the regions with mild ID generally lay along the coast (Fig. 1).
Our results underscore the significant contribution of salt iodization to the iodine status of the women. A study involving 10 countries in Asia and Africa revealed that 26.2% of Philippine households used adequately iodized salt (≥15 ppm), with a higher proportion among those in urban areas (31.5%) than among those in rural areas (20.2%). However, that study pointed out that the relative difference in household access to adequately iodized salt was much more strongly affected by SES than by residence type. In fact, the relative coverage of households with adequately iodized salt in low-SES households was 56% lower than in high-SES households [32].
In the present study, the median UIC was found to be positively correlated to the amount of iodine present in the salt. The same pattern was observed in Indonesia, wherein the median UIC in 13,218 reproductive-age women varied from 154.0 µg/L for those using non-iodized salt to 222.0 µg/L for over-iodized salt users [24]. Likewise, non-pregnant, non-lactating women in Sierra Leone who had iodized salt in the household had significantly higher median UIC levels (217.2 µg/L) than their non-iodized salt user counterparts [23].
This study also showed that educational attainment was correlated to iodine nutritional status. The median UIC increased from the group who had not completed any formal education to those women who had reached a college-level education.
Studies conducted in Australia and Norway showed that women had poor knowledge of matters related to iodine nutrition. Significantly higher knowledge scores were observed among highly educated women and those who had received information about iodine [33], while an assessment among Australian women before the mandatory iodine fortification of all salt used for bread-making was approved showed poor overall knowledge. Less than half of the women identified ID as a cause of mental and physical impairment in fetal development. Furthermore, pregnancy and lactation were not considered to be high-risk periods for ID [34].
The study was limited to an assessment of UIC levels of women who were included in the list of households during the eighth NNS. Whether participants had a previous history of diseases related to iodine nutrition, medications, or supplements was not considered, and their knowledge about the important functions of iodine was not assessed. Nevertheless, this study provided both national and regional assessments of iodine status among childbearing-age women, which can be utilized by health practitioners in planning programs designed for specific localities.
In conclusion, the study observed that while the national estimate of UIC was above the threshold value, pockets of deficiency were nonetheless observed. These areas were primarily in the southern part of the archipelago, and are generally considered to be rural areas where the poor and the poorest reside. Moreover, households with adequately iodized salt had better iodine status than those with levels below the mandated fortification levels. This difference may be attributable to differences in women's educational attainment, since knowledge about the importance of iodine, in general, has been identified as an essential determinant of iodine nutrition. Assessment of the iodine status of these women as they become pregnant, including other factors that may contribute to participants' overall iodine status, is encouraged in order to obtain a more complete understanding of the scope and significance of iodine nutrition before pregnancy.
ACKNOWLEDGMENTS
The authors thank the biochemical researchers and science aides for urine collection, storage, and transport, and the women of childbearing age who participated in the study. This work was presented in abstract form at the 2018 Seoul International Congress of Endocrinology and Metabolism in Seoul, Korea.
References
1. International Council for Control of Iodine Deficiency Disorders. UNICEF. World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva: World Health Organization;2007.
2. Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013; 382:331–337. PMID: 23706508.

3. Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab. 2013; 98:1954–1962. PMID: 23633204.

4. Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutr. 2007; 10:1542–1546. PMID: 18053277.

5. Department of Science and Technology-Food and Nutrition Research Institute (DOST-FNRI). Philippine Dietary Reference Intakes 2015 [Internet]. Taguig: DOST-FNRI;2015. cited 2018 Jul 3. Available from: http://www.fnri.dost.gov.ph/index.php/tools-and-standard/philippine-dietary-reference-intakes-pdri.
6. Perlas LA, Ulanday JRC, Marcos JM, Serafico ME, Desnacido JA, Alibayan MV, et al. Iodine deficiency disorder among Filipino school children, pregnant and lactating women and the elderly 20 years after the Act for Salt Iodization Nationwide Law. J Endocrinol Metab. 2017; 7:86–93.

7. Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005-2010. Thyroid. 2013; 23:927–937. PMID: 23488982.

8. Pan Y, Caldwell KL, Li Y, Caudill SP, Mortensen ME, Makhmudov A, et al. Smoothed urinary iodine percentiles for the US population and pregnant women: National Health and Nutrition Examination Survey, 2001-2010. Eur Thyroid J. 2013; 2:127–134. PMID: 24783051.

9. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011; 21:1081–1125. PMID: 21787128.

10. Mills JL, Buck Louis GM, Kannan K, Weck J, Wan Y, Maisog J, et al. Delayed conception in women with low-urinary iodine concentrations: a population-based prospective cohort study. Hum Reprod. 2018; 33:426–433.

11. Council for International Organizations of Medical Sciences (CIOMS). International ethical guidelines for epidemiological studies. Geneva: CIOMS;2008.
12. Philippine Council for Health Research and Development-Department of Science and Technology. Philippine National Ethical Guidelines for Health Research, 2011. Taguig: Philippine National Health Research System;2011.
13. Department of Science and Technology-Food and Nutrition Research Institute (DOST-FNRI). Philippine Nutrition Facts and Figures 2013: 8th National Nutrition Survey Overview [Internet]. Taguig: DOST-FNRI;2015. cited 2018 Jul 3. Available from: http://enutrition.fnri.dost.gov.ph/assets/uploads/publications/Overview_8thNNS_050416.pdf.
14. Pino S, Fang SL, Braverman LE. Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin Chem. 1996; 42:239–243. PMID: 8595717.

15. Lumley T, Scott AJ. Two-sample rank tests under complex sampling. Biometrika. 2013; 100:831–842.

16. Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004; 151(Suppl 3):U25–U37. PMID: 15554884.

17. Chan S, Kilby MD. Thyroid hormone and central nervous system development. J Endocrinol. 2000; 165:1–8. PMID: 10750030.

18. Glinoer D, Delange F. The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid. 2000; 10:871–887. PMID: 11081254.

19. Delange F. Iodine deficiency as a cause of brain damage. Postgrad Med J. 2001; 77:217–220. PMID: 11264481.

20. Donnay S, Arena J, Lucas A, Velasco I, Ares S. Working Group on Disorders Related to Iodine Deficiency and Thyroid Dysfunction of the Spanish Society of Endocrinology and Nutrition. Iodine supplementation during pregnancy and lactation. Position statement of the working group on disorders related to iodine deficiency and thyroid dysfunction of the Spanish Society of Endocrinology and Nutrition. Endocrinol Nutr. 2014; 61:27–34. PMID: 24035326.

21. Preedy VR, Burrow GN, Watson R. Chapter 62, Iodine deficiency and the brain: an overview. Comprehensive handbook of iodine. Amsterdam: Academic Press;2009. p. 598–606.
22. Department of Science and Technology-Food and Nutrition Research Institute (DOST-FNRI). Philippine Nutrition Facts and Figures 2013: Biochemical Survey [Internet]. Taguig: DOST-FNRI;2015. cited 2018 Jul 3. Available from: http://enutrition.fnri.dost.gov.ph/site/uploads/2013_FaF_Biochemical_Survey.pdf.
23. Rohner F, Wirth JP, Woodruff BA, Chiwile F, Yankson H, Sesay F, et al. Iodine status of women of reproductive age in Sierra Leone and its association with household coverage with adequately iodized salt. Nutrients. 2016; 8:74. PMID: 26848685.

24. Kartono D, Atmarita A, Jahari AB, Soekirman S, Izwardy D. The situation of urinary iodine concentration (UIC) among school age children, women at reproductive age and pregnant women in Indonesia: the analysis of Riskesdas 2013. Gizi Indon. 2016; 39:49–58.
25. Burns K, Yap C, Mina A, Gunton JE. Iodine deficiency in women of childbearing age: not bread alone. Asia Pac J Clin Nutr. 2018; 27:853–859. PMID: 30045431.
26. Land MA, Webster JL, Ma G, Li M, Su'a SA, Ieremia M, et al. Salt intake and iodine status of women in Samoa. Asia Pac J Clin Nutr. 2016; 25:142–149. PMID: 26965773.
27. Edmonds JC, McLean RM, Williams SM, Skeaff SA. Urinary iodine concentration of New Zealand adults improves with mandatory fortification of bread with iodised salt but not to predicted levels. Eur J Nutr. 2016; 55:1201–1212. PMID: 26018655.

28. Kshatri JS, Karmee N, Tripathy RM. Prevalence and predictors of poor iodine nutrition in rural South Odisha: a comparative study between coastal and hilly districts. Natl J Community Med. 2017; 8:41–46.
29. Konrade I, Neimane L, Makrecka M, Strele I, Liepinsh E, Lejnieks A, et al. A cross-sectional survey of urinary iodine status in Latvia. Medicina (Kaunas). 2014; 50:124–129. PMID: 25172607.

30. Wang Z, Zhu W, Mo Z, Wang Y, Mao G, Wang X, et al. An increase in consuming adequately iodized salt may not be enough to rectify iodine deficiency in pregnancy in an iodine-sufficient area of China. Int J Environ Res Public Health. 2017; 14:E206. PMID: 28230748.

31. Costa Leite J, Keating E, Pestana D, Cruz Fernandes V, Maia ML, Norberto S, et al. Iodine status and iodised salt consumption in Portuguese school-aged children: the iogeneration study. Nutrients. 2017; 9:E458. PMID: 28475154.

32. Knowles JM, Garrett GS, Gorstein J, Kupka R, Situma R, Yadav K, et al. Household coverage with adequately iodized salt varies greatly between countries and by residence type and socioeconomic status within countries: results from 10 national coverage surveys. J Nutr. 2017; 147:1004S–1014S. PMID: 28404840.

33. Garnweidner-Holme L, Aakre I, Lilleengen AM, Brantsaeter AL, Henjum S. Knowledge about iodine in pregnant and lactating women in the Oslo area, Norway. Nutrients. 2017; 9:E493. PMID: 28505075.

34. Charlton KE, Yeatman HR, Houweling F. Poor iodine status and knowledge related to iodine on the eve of mandatory iodine fortification in Australia. Asia Pac J Clin Nutr. 2010; 19:250–255. PMID: 20460240.
Fig. 1
Regional iodine status among Filipino women of childbearing age based on median urinary iodine concentration.
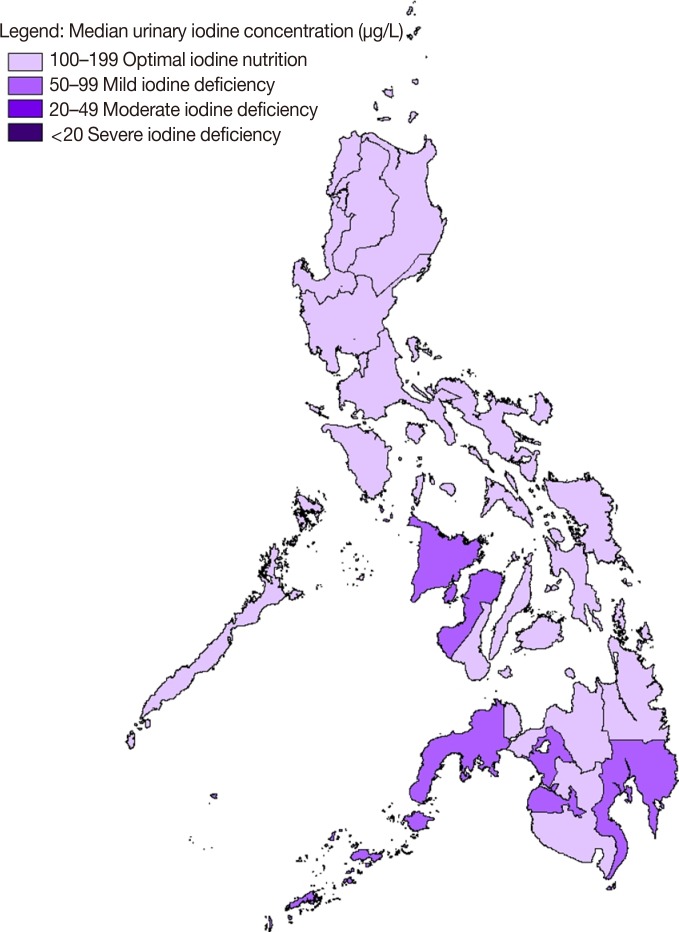
Table 1
Epidemiological Criteria for Assessment of Iodine Nutrition in a Population Based on Median Urinary Iodine Concentration
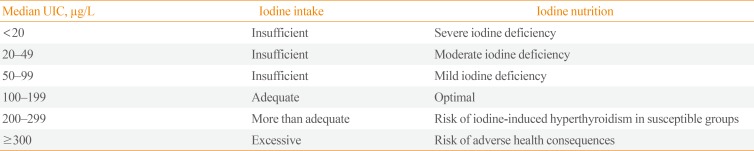
Table 2
Median Urinary Iodine Concentrations and Percentages of Levels <50 and ≥100 µg/L in Filipino Women Aged 15 to 45 Years Old by Region
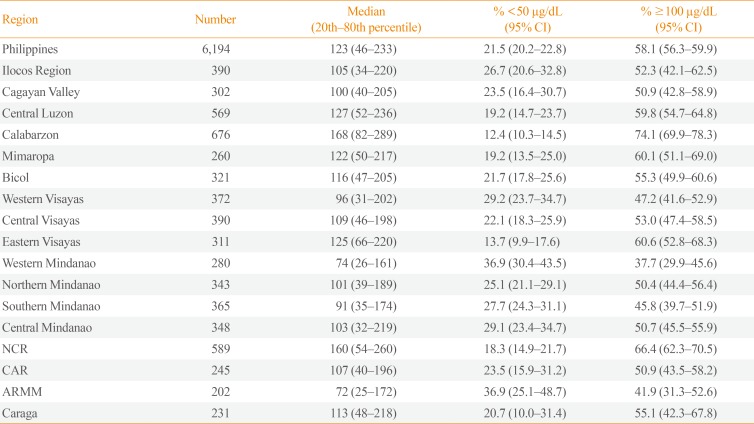
Table 3
Percent Distribution of Urinary Iodine Concentration in Filipino Women of Childbearing Age by Region
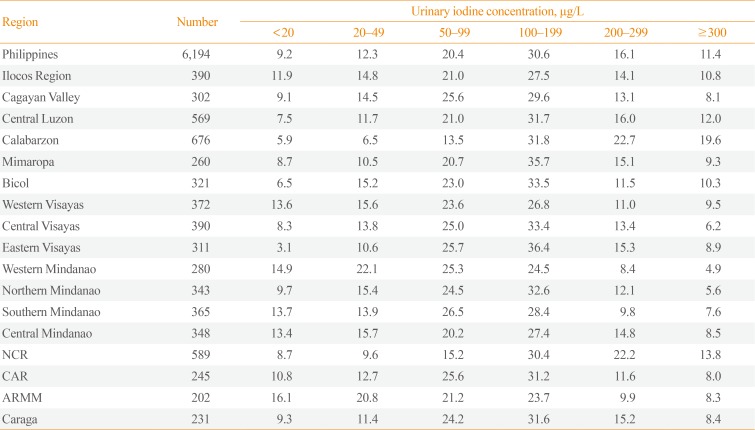
Table 4
Distribution of Urinary Iodine Concentration among Women of Childbearing Age by Place of Residence, Wealth Quintile, Level of Iodine in Salt, and Educational Attainment




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download