Abstract
Background
Methods
Results
Figures and Tables
Fig. 1
Participants disposition to be included in this study. KNHANES, Korea National Health and Nutrition Examination Survey.
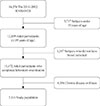
Table 1
Demographic and Clinical Characteristics of Korean Population, Aged 19 Years and Older, in 2011 to 2012 Korea National Health and Nutrition Examination Survey
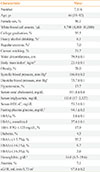
Values are expressed as median (range) or mean±SEM.
HDL-C, high density lipoprotein cholesterol; HbA1c, hemoglobin A1c; FPG, fasting plasma glucose; eGFR, estimated glomerular filtration rate.
aHeavy alcohol drinking ≥×4 alcoholic drinks/week; bRegular exercise ≥×5 exercise/week; cBody mass index ≥25 kg/m2; dSystolic blood pressure ≥140 mm Hg without anti-hypertensive medication; eDiastolic blood pressure ≥90 mm Hg without anti-hypertensive medication; fFasting plasma glucose ≥126 mg/dL without anti-diabetes medication; gHemoglobin <13 g/dL in men and <12 g/dL in women.
Table 2
Age, Sex, and Age- and Sex-Adjusted Demographic and Clinical Characteristics According to the Quartile of WBC Level

Values are expressed as mean±SEM.
WBC, white blood cell; BP, blood pressure; HDL-C, high density lipoprotein cholesterol; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate.
aHeavy alcohol drinking ≥×4 alcoholic drinks/week; bRegular exercise ≥×5 exercise/week; cBody mass index ≥25 kg/m2; dSystolic BP ≥140 mm Hg without anti-hypertensive medication; eDiastolic BP ≥90 mm Hg without anti-hypertensive medication; fFPG ≥126 mg/dL without anti-diabetes medication; gHemoglobin <13 g/dL in men and <12 g/dL in women.
Table 3
Hemoglobin A1c (%) According to the Quartile of WBC Level

Values are expressed as mean±SEM.
WBC, white blood cell; FPG, fasting plasma glucose.
aModel 1: unadjusted; bModel 2: adjusted for age, sex, and FPG; cModel 3: adjusted for college graduation, smoking history (never, past, and current smoking), waist circumference, the presence of hypertension, serum total cholesterol, serum triglyceride, the presence of anemia, and variables in model 2; dModel 4, model 3 after exclusion of FPG ≥126 mg/dL (n=204).
Table 4
The Proportions of HbA1c ≥5.7%, ≥6.1%, and ≥6.5% (%) According to the Quartile of WBC Level

Values are expressed as mean±SEM.
HbA1c, hemoglobin A1c; WBC, white blood cell.
aModel 1: unadjusted; bModel 2: adjusted for age, sex, and fasting plasma glucose (FPG); cModel 3: adjusted for college graduation, smoking history (never, past, and current smoking), waist circumference, the presence of hypertension, serum total cholesterol, serum triglyceride, the presence of anemia, and variables in model 2; dModel 4, model 3 after exclusion of FPG ≥126 mg/dL (n=204).
Table 5
ORs of White Blood Cell Quartiles for the Risks of HbA1c Levels of ≥5.7%, ≥6.1%, and ≥6.5%
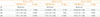
Covariates: age, sex, and fasting plasma glucose, college graduation, smoking history (never, past, and current smoking), waist circumference, the presence of hypertension, serum total cholesterol, serum triglyceride, the presence of anemia.
OR, odds ratio; HbA1c, hemoglobin A1c; CI, confidence interval.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download