INTRODUCTION
Thyrotoxicosis refers to a clinical syndrome of excess circulating thyroid hormone, irrespective of the source, which affects many organ systems [
1]. The clinical symptoms and signs of thyrotoxicosis are fatigue, anxiety, palpitations, sweating, heat intolerance, anxiety, disturbed sleep, weight loss, tachycardia, and tremor of the extremities [
23]. These clinical manifestations are relatively nonspecific and can vary depending on factors such as the patient's age, sex, comorbidities, and the duration and cause of the disease [
45]. This variety of nonspecific symptoms and signs makes it difficult to diagnose or assess disease status based on symptoms and signs alone. Moreover, when deciding how to treat patients diagnosed with thyrotoxicosis, the physician needs to assess the clinical severity of disease and its predicted response to the available treatments. Because the clinical severity of thyrotoxicosis does not always reflect the magnitude of the elevation in thyroid hormone levels [
6], it would be useful to have a method to assess clinical status and the response to various treatments.
Previous attempts have been made to develop instruments to evaluate the clinical symptoms and signs of thyrotoxicosis. Crooks et al. [
7] described a diagnostic index that scores the presence or absence of various signs and symptoms of thyrotoxicosis for the purpose of establishing a diagnosis. In 1965, Sir Edward Wayne described a scale to improve the accuracy of diagnosis of hyperthyroidism [
8]. Wayne's index reports a score ranging from −25 to +45, where a score <11 is defined as “euthyroidism” and a score >19 suggests “toxic hyperthyroidism” [
9]. Klein et al. [
10] developed a hyperthyroidism symptom scale (HSS) and used it in 10 patients with untreated Graves' disease (GD). In that study, HSS was compared at three different phases: during hyperthyroidism at the time of diagnosis (phase 1), after 2 weeks of treatment with propranolol hydrochloride (phase 2), and in a euthyroid state after 6 months of treatment with propylthiouracil (phase 3). The mean scores decreased significantly between phases 1 and 2, paralleling the decreases in the symptoms and appeared to be sensitive to changes in both adrenergic stimulation and the metabolic effects of thyroid hormone as reflected by the sequential fall in scores between phases 2 and 3.
Because thyroid function tests (TFTs) are currently used to confirm thyrotoxicosis, the evaluation of the clinical status of patients using the HSS might be regarded as less important than previously [
11]. However, it remains a helpful tool for diagnosis and assessing the response to treatment. Because of these advantages of the HSS, and because there is no Korean-language tool to evaluate the clinical status of patients with thyrotoxicosis, our aim was to develop a Korean version of the HSS (K-HSS) and evaluate its validity and reliability.
METHODS
HSS translation
The original version of HSS was initially translated into Korean by a Korean endocrinologist. Subsequently, this Korean version was back-translated by a bilingual endocrinologist who was not familiar with the original version. The resulting revision was reviewed and amended by a translation committee consisting of three Korean endocrinologists. Thus, the final draft version was the linguistic equivalent of the original.
Anthropometric and biochemical measurements
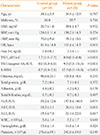
Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, with the subject in light clothing and without shoes. Body mass index was calculated as weight divided by the height squared (expressed in kilograms per square meter). Blood pressure and heart rate were measured on the right arm with the subject in a seated position. Serum glucose was measured using a Hitachi 747 chemistry analyzer (Hitachi, Tokyo, Japan). Serum total protein, albumin, total bilirubin, alkaline phosphatase, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured with an autoanalyzer (TBA-200FR, Toshiba, Tokyo, Japan). For TFT, concentrations of serum free thyroxine (T4; DiaSorin, Saluggia, Italy) and thyroid stimulating hormone (TSH; CIS Bio International, Gif-sur-Yvette, France) were measured using immunoradiometric assays. The free T4 assay had an analytical sensitivity of 0.05 ng/dL, while that for TSH had an analytical sensitivity of 0.04 mIU/L and a functional sensitivity of 0.07 mIU/L. The reference ranges for free T4 and TSH were 0.89 to 1.79 ng/dL and 0.3 to 4.0 mIU/L, respectively. Thyrotoxicosis was defined based on the results of the TFT: i.e., overt thyrotoxicosis was defined as high free T4 and low TSH, and subclinical thyrotoxicosis as normal free T4 and low TSH.
Participants
Patients with thyrotoxicosis were recruited from the outpatient clinic of the endocrinology department at Seoul National University Bundang Hospital (SNUBH). We included 30 patients aged 15 to 60 years with newly diagnosed or recurrent thyrotoxicosis. Two patients were excluded from the study because of poor compliance, so only the data from the other 28 patients were used for the final analysis. Of these, 25 had GD and three had transient thyrotoxicosis due to thyroiditis. Patients with GD were prescribed a specific dose of antithyroid drugs (ATDs) as decided by the physician. Patients with transient thyrotoxicosis were reassured that their symptoms and signs were benign and self-limiting. Patients taking a β-blocker for control of symptoms such as palpitations and tremor were advised to reduce or discontinue the dose of β-blocker if the symptoms improved. Patients with thyrotoxicosis caused by a toxic nodular goiter were also excluded because this condition requires treatment options other than ATDs, which have an inadequate therapeutic effect. Ten healthy volunteers who were checked to confirm that they had no history of thyroid disease were also included as a control group. All subjects participating were informed about the study and completed written informed consent. The study was approved by the Ethical Review Board of SNUBH (IRB No. B-1709/418-102).
Procedures
Anthropometric data such as height, weight, blood pressure, and heart rate were measured at the first visit of those patients with newly detected or recurrent thyrotoxicosis who agreed to participate in this study. They also underwent blood tests including TFT and tests to identify the cause of the thyrotoxicosis (e.g., autoantibodies, thyroid scan). To assess clinical hyperthyroidism, the endocrinologist in charge of this study evaluated the patients using the K-HSS [
12]. The information needed to rate each item was obtained by the endocrinologist through the history taking and physical examination. It was possible to recruit all patients from outpatient clinics of one endocrinologist with a relatively small study of 40 participants. Therefore, one endocrinologist, who was in charge of this study, was responsible for the evaluation of all patients. After 1 to 2 weeks, the patients visited the clinic to confirm the results of the previous tests and to start treatment based on the cause of their thyrotoxicosis. All patients were followed-up every 1 to 2 months, at which times they underwent blood tests including TFT. In the patients with GD, the ATD dose was adjusted as necessary. At each visit, anthropometric data were collected and vital signs were measured. The same endocrinologist evaluated the clinical thyrotoxicosis status using K-HSS.
Healthy volunteers were recruited as a control group through an official announcement in SNUBH. The controls visited the hospital on the same schedule as the patients with thyrotoxicosis. They also underwent the same blood tests and met the endocrinologist at each visit and participated in the evaluation of K-HSS.
Assessment items in K-HSS
The original HSS gives scores from 0 to 40, where higher scores indicate more severe disease. It consists of a 10-item scale that rates nervousness, diaphoresis, heat intolerance, motor activity, tremor, weakness, hyperdynamic precordium, diarrhea, weight loss/appetite, and overall function. This structure was retained in the Korean version. The information needed to score each item was obtained through history taking and physical examination. The intensity of each item can be rated on a subscale from 0 to 4 points and the item scores were summed to obtain the overall score.
Statistical analysis
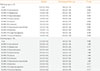
Data were expressed as mean±standard deviation (SD). To compare variables between the patient and control groups, we used the Mann-Whitney test (non-parametric data such as TSH, TSH receptor antibody [Ab] and K-HSS) or Student t test (for the remaining parametric data) for continuous variables and the chi-square test for categorical variables. The associations of free T4 or TSH levels and K-HSS scores at baseline were evaluated by ordinary least-square regression analysis. To compare the variables at baseline and follow-up, we used the Wilcoxon signed rank test or paired t test. One-way analysis of variance or Kruskal-Wallis test was used to compare thyroid function and K-HSS scores between three groups: posttreatment patients who remained subclinically thyrotoxic, patients who became euthyroid after treatment and the control group at follow-up. Cronbach's α coefficient was calculated to evaluate the internal consistency of K-HSS. A two-tailed P value of <0.05 was considered significant. IBM SPSS for Windows version 22.0 (IBM Co., Armonk, NY, USA) was used for the data analyses.
DISCUSSION
In this study, K-HSS scores were positively associated with the baseline serum free T4 levels for all subjects, but not for thyrotoxic patients. For the patient group, the K-HSS scores decreased significantly after 3 months of observation or treatment with ATDs. In contrast, there were no significant changes in the scores for the control group even when compared item by item. The reliability of the K-HSS as assessed by Cronbach's α was also confirmed to be high.
This study showed a significant positive correlation between serum free T4 levels and K-HSS scores in all subjects at their first visit. However, there was no significant correlation between them in patients group. There is little information available about the correlation between the degree of clinical thyrotoxicosis and the results of TFT. One study that evaluated the severity of clinical thyrotoxicosis using the original HSS reported that in 25 patients with untreated GD the HSS was not associated with thyroid function assessed by free T4 index or serum T3 measured by radioimmunoassay [
13]. Kolawole et al. [
14] also reported that there was no significant association between laboratory T3, T4, and TSH values and HSS in 15 patients with newly diagnosed and untreated thyrotoxicosis. Therefore, the results for patient group from this study were consistent with previous reports which suggested the severity of peripheral endocrine symptoms, as measured by the K-HSS (HSS) was not linearly related to thyroid hormone levels such as T3 and free T4. According to the previous report, this might be due to potential variables such as cellular responsiveness to thyroid hormones or catecholamines on account of the difference in the degree of expression of thyroid hormone receptor or the density of β-adrenergic receptor [
13]. In addition, given that initial free T4 and K-HSS (HSS) scores were inevitably similar to each other in untreated patients with thyrotoxicosis, it could be one of the reason for non-significant correlation between free T4 and K-HSS (HSS) that they did not include enough number of subjects to confirm the correlation between them. Although not shown in the results of this study, the correlation between free T4 and K-HSS before and after the treatment in thyrotoxic patients was significant (B=3.245, 95% confidence interval, 2.161 to 4.330;
P<0.001) (lineal model of generalized estimating equation analysis). In 28 patients in our study, thyroid function gradually improved with time or administration of ATDs. After approximately 3 months of observation or treatment, six patients were euthyroid and 19 patients were subclinically thyrotoxic. We excluded three patients who remained overtly thyrotoxic from the analysis because the purpose of this study was to analyze the changes in K-HSS scores accompanying an improvement in thyroid function induced with medication. The K-HSS scores decreased significantly with improved thyroid function. These results adequately validate the K-HSS and suggest that it could be useful for predicting the response to treatment in thyrotoxic patients. However, although there was no significant difference in free T4 between the posttreatment patients group and the control group at follow-up, K-HSS scores differed significantly between these groups. Considering that the majority of the posttreatment patient group were subclinically thyrotoxic, this could be interpreted to indicate that the K-HSS scores are related not only to free T4 levels but also to overall thyroid function. To evaluate this possibility, we divided the posttreatment patient group into two subgroups according to their thyroid function (subclinically thyrotoxic or euthyroid), and compared K-HSS scores between the subclinically thyrotoxic, euthyroid posttreatment patients and the controls at follow-up. The three patients who remained overtly thyrotoxic were excluded. There was no significant difference in the K-HSS scores of subclinically thyrotoxic and euthyroid subjects from the posttreatment patient group. As indicated by the box plots presented in
Fig. 2, the euthyroid posttreatment patient group was relatively small (
n=6) and showed a broad distribution of K-HSS scores. Therefore, further evaluation is needed in a larger number of patients to clarify whether the K-HSS scores reflects free T4 as well as overall thyroid function.
The K-HSS is composed of 10 items, as is the original HSS, and most of these could be answered by patients after self-evaluation of their condition. Most of the items showed significant differences between scores at baseline and follow-up in thyrotoxic patients, and only item 9 showed marginal significant difference (
P=0.050). This item concerns the degree of appetite and body weight, and could have been confusing because these variables are fairly subjective and the criteria for scoring were not clear. To confirm the internal consistency of K-HSS, we calculated Cronbach's α coefficient [
15]. There are different definitions of the acceptable values of α, ranging from 0.70 to 0.95 [
16]: a high value of α (>0.90) may suggest redundancies and indicate that the test should be shortened [
17]. In the current study, α was 0.86, high enough to confirm the reliability of the K-HSS.
To the best of our knowledge, this is the first report to validate a Korean-language scoring scale that evaluates the clinical status of thyrotoxicosis. Moreover, this study was a prospective study that followed up the changes in K-HSS scores after observation or administration of ATDs. Although a remarkable downgrading of the importance of clinical aspects of hyperthyroidism has occurred as implementation of TFT becomes more common [
18], if a simple assessment of K-HSS scores could provide a clue to predict thyroid function it could be useful information for many aspects of the treatment of patients suspected of or diagnosed with thyrotoxicosis. For example, in patients with GD, their K-HSS scores could be used to estimate the response to ATDs and to prompt those patients who relapse after discontinuation of ATDs to visit the hospital as soon as possible. Furthermore, it could be used to estimate whether the dose of thyroid hormone was appropriate in patients with thyroid cancer undergoing TSH-suppressive therapy, who develop an iatrogenic subclinical thyrotoxicosis, to prevent the recurrence or progression of thyroid cancer with further studies on the thyroid cancer patients [
19]. In addition to these clinical aspects, an index that could objectively assess the symptoms of thyrotoxicosis might be helpful for research.
This study had some limitations. The first was the failure to analyze changes after administration of β-blockers, which are commonly administered during thyrotoxicosis. This was because, according to the study protocol, the follow-up blood tests and K-HSS were performed 1 to 2 months after patients took the β-blocker, and the patients were trained to stop or reduce the dosage of the medication with symptom improvement. Next, the K-HSS was validated only in a single group recruited from a single center and assessed by one endocrinologist. As previously mentioned, there are some subjective items that could be given different scores by different endocrinologists, so analysis of multicenter patient groups may be required to confirm the results.
In conclusion, the K-HSS could be an easy-to-use tool for assessing the severity of thyrotoxicosis and the response to treatments such as ATDs. Based on the results from our study, the K-HSS could be used in research and clinical settings to enhance clinical understanding and decision-making, but not as the sole basis for making a clinical diagnosis.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download