Abstract
Core needle biopsy (CNB) was introduced as an alternative diagnostic tool to fine-needle aspiration (FNA), and is increasingly being used in the preoperative assessment of thyroid nodules. CNB provides a definitive diagnosis in most cases, but it sometimes may be inconclusive. CNB has the advantage of enabling a histologic examination in relation to the surrounding thyroid tissue, immunohistochemistry, and molecular testing that can provide a more accurate assessment than FNA in selected cases. Nevertheless, CNB should be performed only by experienced experts in thyroid interventions to prevent complications because CNB needles are larger in caliber than FNA needles. As recent evidence has accumulated, and with improvements in the technique and devices for thyroid CNB, the Korean Society of Thyroid Radiology released its 2016 thyroid CNB guidelines and the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group published a consensus statement on the pathology reporting system for thyroid CNB in 2015. This review presents the current consensus and recommendations regarding thyroid CNB, focusing on indications, complications, and pathologic classification and reporting.
Fine-needle aspiration (FNA) cytology is the gold-standard diagnostic method for the preoperative diagnosis of thyroid nodules [12]. Although thyroid aspiration cytology using a large needle was initially introduced in the 1920s, FNA using a 23- to 27-gauge needle has been a mainstream diagnostic modality in the triage of thyroid nodules since the 1980s in most countries because of its diagnostic accuracy and safety [34567]. Core needle biopsy (CNB) of the thyroid was first introduced as an alternative to FNA in the 1990s [4] and is now widely used for the preoperative examination of thyroid nodules in Korea, with excellent results, because of advances in core needle devices (thinner needles and automatic devices), biopsy technology (new sampling technique) and ultrasound machine (high resolution) [8]. In this review, we present an overview of the indications, complications, and pathologic classification of thyroid CNB.
As evidence has accumulated regarding thyroid CNB, the Korean Society of Thyroid Radiology (KSThR) released its 2016 thyroid CNB guidelines [9]. These guidelines include recommendations regarding indications and complications, and are summarized in Table 1. A consensus statement on the pathology reporting system for thyroid CNB was published by the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group in 2015 [10]. The six categorical diagnoses used in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) were retained in the reporting system for thyroid CNB in order to ensure effective communication between pathologists and clinicians, with less likelihood of misinterpreting the pathologic results [110].
The current thyroid guidelines of the American Association of Clinical Endocrinologists/American College of Endocrinology/Associazione Medici Endocrinologi, British Thyroid Association, and KSThR permit the limited use of CNB for thyroid nodules, whereas the American Thyroid Association does not recommend CNB [2911].
The most widely accepted indications for CNB in the literature include previous FNA results of non-diagnostic [12131415] and atypia of undetermined significance [151617]. The tissue obtained by CNB provides more abundant material than FNA cytology, especially in cases with marked sclerosis and calcification. A recent meta-analysis of published data showed that the non-diagnostic and inconclusive rates of CNB were 5.5% (95% confidence interval [CI], 2.2% to 8.7%) and 8.0% (95% CI, 4.4% to 11.5%), respectively, whereas the non-diagnostic and inconclusive rates of FNA were 22.6% (95% CI, 12.2% to 33.0%) and 40.2% (95% CI, 25.1% to 55.3%), respectively [18]. In another meta-analysis of thyroid nodules with initially non-diagnostic FNA results, the non-diagnostic rate (6.4%; 95% CI, 3.3% to 16.1%) of follow-up CNB was significantly lower than that of repeated FNA (36.5%; 95% CI, 29.9% to 43.1%) [13]. In large cohort CNB studies, the false-negative rates of CNB ranged from 1% to 3% [192021].
Although CNB may not be the method of choice for all thyroid nodules, previous studies have demonstrated the advantages of histologic diagnoses made using CNB specimens over the cytological diagnosis using FNA for several specific diseases. Malignant lymphoma, medullary thyroid carcinoma, anaplastic thyroid carcinoma, and parathyroid lesions can be confirmatively diagnosed with CNB based on the histologic morphology in conjunction with immunohistochemistry [222324]. While calcified nodules and degenerating nodules are often diagnosed as unsatisfactory with FNA because of the aspiration of acellular or paucicellular material, scant cellular nodules can be diagnosed as specific disease entities with CNB (Fig. 1) [252627].
Follicular-patterned lesions include nodular hyperplasia, follicular adenoma, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), the follicular variant of papillary thyroid carcinoma (PTC), and follicular thyroid carcinoma. While FNA and CNB cannot differentiate follicular adenoma from follicular carcinoma and NIFTP from the invasive encapsulated follicular variant of PTC because vascular or capsular invasion cannot be assessed without surgery, it is possible to divide these follicular-patterned lesions into almost certainly benign non-neoplastic lesions and follicular newoplasms with a risk of malignancy. NIFTP and the follicular variant of PTC are diagnosed based on nuclear features in conjunction with architectural atypia showing a follicular growth pattern. For the diagnosis of follicular neoplasm with CNB, it is recommended that the tissue sampling of CNB should include tumor tissue, the tumor capsule, and adjacent normal parenchyma (Fig. 2).
A few studies have suggested that CNB is valuable as a first-line tool for diagnosing initially detected thyroid nodules [2128]. Suh et al. [21] reported that CNB had a non-diagnostic rate of 1.3% and an inconclusive result rate of 5.9%, a high diagnostic accuracy of 97.6%, and a complication rate of 0.2%.
The current thyroid guidelines suggest that CNB is a safe, tolerable procedure [1529]. The complication rate is also acceptable (0% to 4.1%), with a low rate of major complications (0% to 1.9%). Recently a large-population single-center study showed no procedure-related deaths, and low rates of major (0.06%) and minor (0.79%) complications [30]. To minimize complications, CNB should be performed by well-trained doctors under real-time ultrasound monitoring. Moreover, knowledge of the neck anatomy, anatomical variations, and potential complications is also required for the safe performance of CNB [31].
The pathology reporting system of CNB is based on the six TBSRTC categories [10] and is summarized in Table 2. There is only one difference in terminology between the CNB reporting system and TBSRTC. The diagnostic category III for CNB is referred to as an “indeterminate lesion,” which corresponds to “atypia of undetermined significance” or “follicular lesion of undetermined significance” in TBSRTC. Subclassification based on the cytologic and/or architectural atypia in category III and IV was encouraged to enhance communication with clinicians and pathologists. Each diagnostic category in the TBSRTC has an implied risk of malignancy and clearly recommended clinical management steps regarding the triage of the patient for clinical follow-up, repeated FNA, or surgery. However, only limited data have been reported regarding the risk of malignancy in each diagnostic category of CNB.
Thyroid CNB has been used as an alternative second option to FNA in patients with a previous non-diagnostic or indeterminate diagnosis, and is now suggested as a first-line tool for the diagnosis of thyroid nodule in selected cases. Recent studies have provided evidence for the efficacy and safety of thyroid CNB. Although the current thyroid CNB guidelines and pathologic reporting system are now widely available and affordable, further validation studies should be continued.
ACKNOWLEDGMENTS
This study was partially supported by a grant (HI16C2013) of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
References
1. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017; 27:1341–1346. PMID: 29091573.

2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.

3. Jung CK, Hong S, Bychkov A, Kakudo K. The use of fine-needle aspiration (FNA) cytology in patients with thyroid nodules in Asia: a brief overview of studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med. 2017; 51:571–578. PMID: 29073758.

4. Baek JH. Current status of core needle biopsy of the thyroid. Ultrasonography. 2017; 36:83–85. PMID: 28301922.

5. Pitman MB, Abele J, Ali SZ, Duick D, Elsheikh TM, Jeffrey RB, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:407–424. PMID: 18478608.

6. Silverman JF, West RL, Finley JL, Larkin EW, Park HK, Swanson MS, et al. Fine-needle aspiration versus large-needle biopsy or cutting biopsy in evaluation of thyroid nodules. Diagn Cytopathol. 1986; 2:25–30. PMID: 3720480.

7. Wang C, Vickery AL Jr, Maloof F. Needle biopsy of the thyroid. Surg Gynecol Obstet. 1976; 143:365–368. PMID: 959956.
8. Novoa E, Gurtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck. 2012; 34:1497–1503. PMID: 22127851.

9. Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, et al. Core needle biopsy of the thyroid: 2016 Consensus Statement and Recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017; 18:217–237. PMID: 28096731.

10. Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015; 49:288–299. PMID: 26081825.

11. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract. 2016; 22:622–639. PMID: 27167915.
12. Yeon JS, Baek JH, Lim HK, Ha EJ, Kim JK, Song DE, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013; 268:274–280. PMID: 23525204.

13. Suh CH, Baek JH, Kim KW, Sung TY, Kim TY, Song DE, et al. The role of core-needle biopsy for thyroid nodules with initially nondiagnostic fine-needle aspiration results: a systematic review and meta-analysis. Endocr Pract. 2016; 22:679–688. PMID: 27176143.

14. Choi SH, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol. 2014; 24:2819–2826. PMID: 25038860.

15. Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012; 22:468–475. PMID: 22304417.

16. Choi YJ, Baek JH, Suh CH, Shim WH, Jeong B, Kim JK, et al. Core-needle biopsy versus repeat fine-needle aspiration for thyroid nodules initially read as atypia/follicular lesion of undetermined significance. Head Neck. 2017; 39:361–369. PMID: 27704650.

17. Park KT, Ahn SH, Mo JH, Park YJ, Park DJ, Choi SI, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck. 2011; 33:160–165. PMID: 20848434.

18. Suh CH, Baek JH, Lee JH, Choi YJ, Kim KW, Lee J, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine. 2016; 54:315–328. PMID: 27220941.

19. Paja M, del Cura JL, Zabala R, Corta I, Lizarraga A, Oleaga A, et al. Ultrasound-guided core-needle biopsy in thyroid nodules. A study of 676 consecutive cases with surgical correlation. Eur Radiol. 2016; 26:1–8. PMID: 25956937.

20. Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim JH, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012; 22:1564–1572. PMID: 22415411.

21. Suh CH, Baek JH, Lee JH, Choi YJ, Kim JK, Sung TY, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. 2016; 26:395–403. PMID: 26651390.

22. Ha EJ, Baek JH, Lee JH, Kim JK, Song DE, Kim WB, et al. Core needle biopsy could reduce diagnostic surgery in patients with anaplastic thyroid cancer or thyroid lymphoma. Eur Radiol. 2016; 26:1031–1036. PMID: 26201291.

23. Ha EJ, Baek JH, Na DG, Kim JH, Kim JK, Min HS, et al. The role of core needle biopsy and its impact on surgical management in patients with medullary thyroid cancer: clinical experience at 3 medical institutions. AJNR Am J Neuroradiol. 2015; 36:1512–1517. PMID: 25929882.

24. Lee SH, Park GS, Jung SL, Kim MH, Bae JS, Lim DJ, et al. Core-needle biopsy for the preoperative diagnosis of follicular neoplasm in thyroid nodule screening: a validation study. Pathol Res Pract. 2016; 212:44–50.

25. Ha EJ, Baek JH, Lee JH, Lee HY, Song DE, Kim JK, et al. A focal marked hypoechogenicity within an isoechoic thyroid nodule: is it a focal malignancy or not? Acta Radiol. 2015; 56:814–819. PMID: 24938659.

26. Ha EJ, Baek JH, Lee JH, Song DE, Kim JK, Shong YK, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid. 2013; 23:703–708. PMID: 23544697.

27. Ha EJ, Baek JH, Lee JH, Kim JK, Kim JK, Lim HK, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol. 2014; 24:1403–1409. PMID: 24604217.

28. Trimboli P, Nasrollah N, Guidobaldi L, Taccogna S, Cicciarella Modica DD, Amendola S, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. 2014; 12:61. PMID: 24661377.

29. Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008; 5:6. PMID: 18394201.

30. Ha EJ, Baek JH, Lee JH, Kim JK, Choi YJ, Sung TY, et al. Complications following US-guided core-needle biopsy for thyroid lesions: a retrospective study of 6,169 consecutive patients with 6,687 thyroid nodules. Eur Radiol. 2017; 27:1186–1194. PMID: 27311538.

31. Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015; 16:749–766. PMID: 26175574.

Fig. 1
Examples of core needle biopsy (CNB) in thyroid nodules with initial non-diagnostic fine-needle aspiration cytology. (A) On the ultrasound findings of case 1, there is a well-defined hypoechoic solid nodule with rim calcification. (B) CNB of the nodule shows scanty cellular and sclerotic nodules (H&E stain, ×40). (C) A high-power view shows the typical histologic features of papillary carcinoma (H&E stain, ×1,000). (D) An ultrasound image from case 2 shows a 1.5-cm, ill-defined hypoechoic solid nodule with macrocalcifications. (E) CNB shows marked calcification sclerosis, and focally follicular proliferative lesions (H&E stain, ×40). (F) A high-power view of the follicular lesion shows the histologic findings of papillary carcinoma (H&E stain, ×1,000).
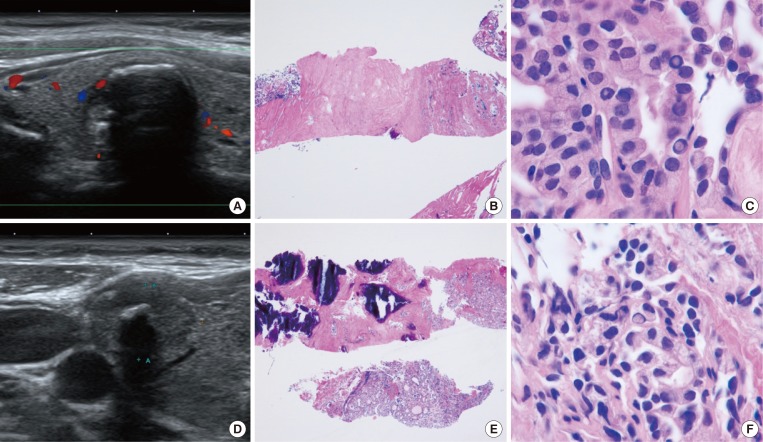
Fig. 2
Current core needle biopsy technique. (A) The specimen notch shown in ultrasound should include the tumor tissue, tumor capsule, and adjacent normal parenchyma. (B) Histologic examination of the specimen shows the tumor tissue with a microfollicular growth pattern, tumor capsule, and surrounding thyroid tissue (H&E stain, ×40, upper; ×400, lower). This case should be diagnosed as a follicular neoplasm.
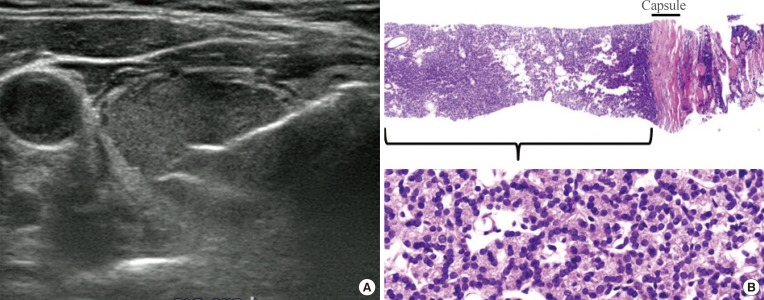
Table 1
Consensus Statement and Recommendations on Thyroid CNB from the Korean Society of Thyroid Radiology [9]
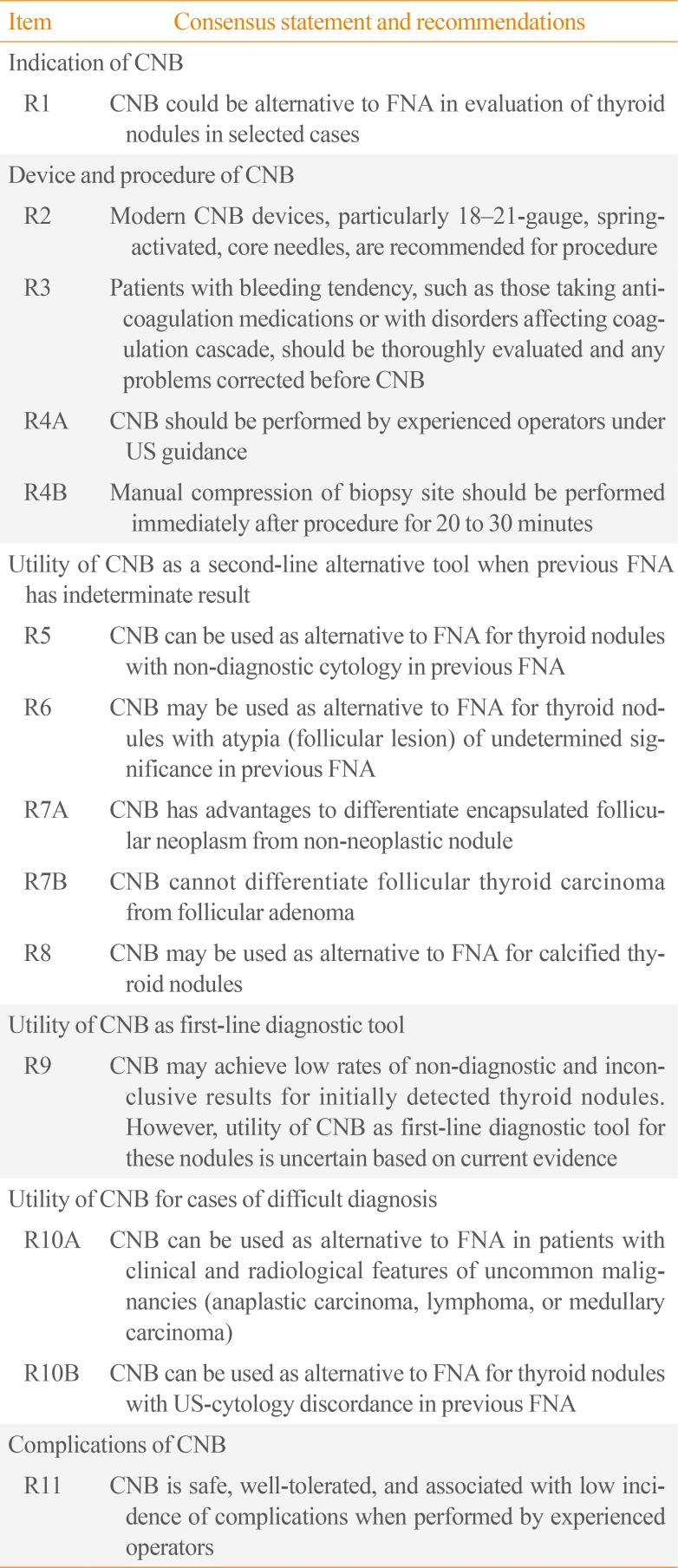
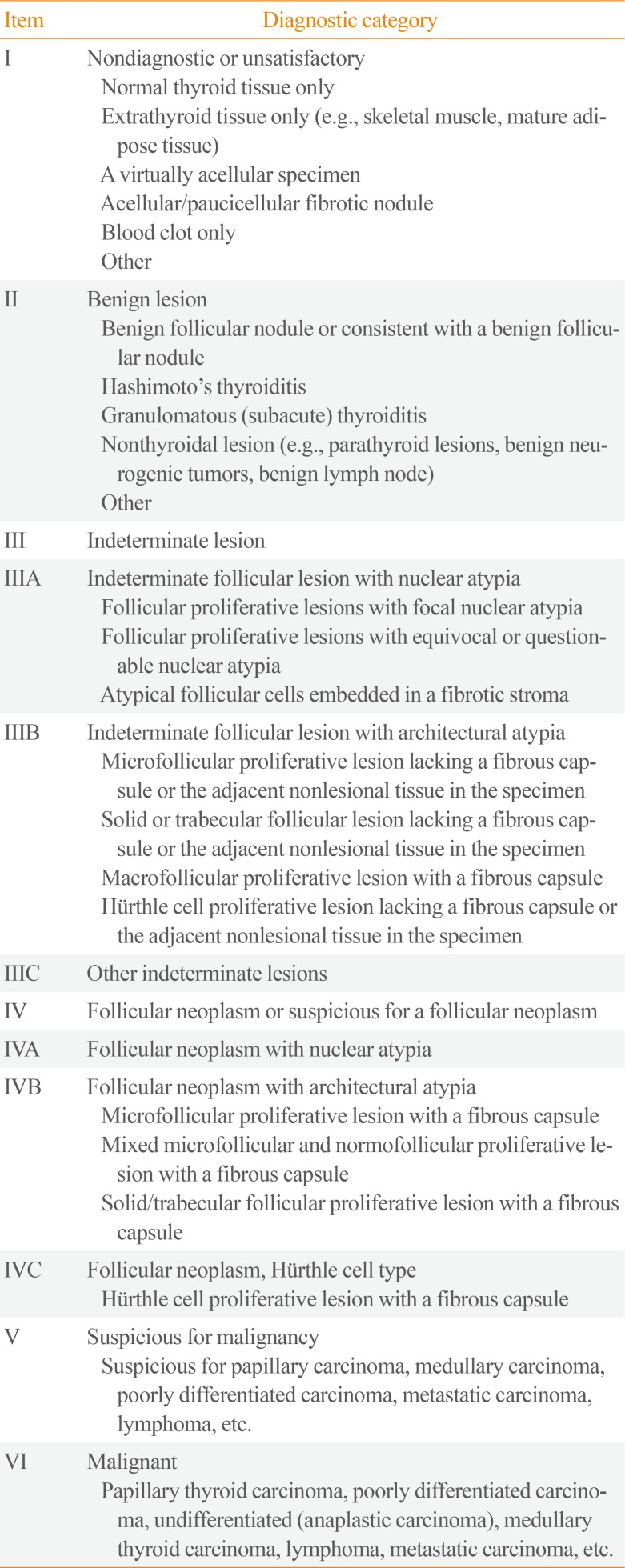
Table 2
Diagnostic Categories of Thyroid Core Needle Biopsy from the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group [10]




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download