Abstract
Background
Glucagon-like peptide 1 (GLP-1), an incretin hormone well known for its glucose-lowering effect, was recently reported to exert an anabolic effect on bone. Although the exact mechanism is not known, it likely involves the GLP-1 receptor (GLP-1R), which is expressed in some osteoblastic cell lines. Adipose-derived stem cells (ADSCs) have mesenchymal stem cell-specific characteristics, including osteoblastic differentiation potential. We evaluated the expression of GLP-1R during osteogenic differentiation of ADSCs.
Methods
ADSCs were isolated from subcutaneous adipose tissue obtained from three male donors during plastic surgery and were subjected to osteogenic induction. Mineralization was assessed by Alizarin Red staining on day 21. Expression of alkaline phosphatase (ALP), osteocalcin (OC), and GLP-1R was measured by real-time polymerase chain reaction in triplicate for each patient on days 0, 7, 14, and 21. Target mRNA expression levels were normalized to that of β-actin.
Results
ADSCs were fibroblast-like in morphology, adhered to plastic, and had multipotent differentiation potential, as assessed using specific antigen markers. The osteogenic markers ALP and OC were notably upregulated at 21 days. Osteogenic differentiation resulted in a time-dependent increase in the expression of GLP-1R (P=0.013).
Glucagon-like peptide 1 (GLP-1) is a 30-amino-acid incretin hormone produced by intestinal L-cells. Although it is well known to exert beneficial effects by lowering postprandial glucose levels, GLP-1 has been reported to have multiple other functions, including modulation of cell proliferation, differentiation, and apoptosis in various tissues [1]. Many recent studies reported an anabolic effect of GLP-1 on bone, with one study showing that GLP-1 reversed hyperlipidemia-related osteopenia in a rat model [2]. Another study reported an insulin-independent anabolic effect of GLP-1 in an insulin-resistant rat model [3]. However, the exact mechanism of this effect has not been established. Results concerning expression of the GLP-1 receptor (GLP-1R) in osteoblastic cells are inconsistent. GLP-1R expression has been reported in various osteoblastic cell lines, albeit at differing levels [4,5]. Adipose-derived stem cells (ADSCs) are reported to be multipotent and capable of osteogenic differentiation [6,7,8], and studies suggest that they could be an abundant, accessible, and replenishable cell source for bone cell therapy applications [9,10]. In an animal study, critical-size mouse calvarial defects were healed using scaffolds seeded with ADSCs [11].
To our knowledge, no studies have described the expression of GLP-1R in ADSCs. The aim of this study was to evaluate the expression of GLP-1R during the osteogenic differentiation of ADSCs.
ADSCs were isolated from subcutaneous abdominal adipose tissue obtained from three male donors (mean age, 40 years) during plastic surgery. Body mass indices of the donors were 22.6, 26, and 23.6 kg/m2. They had received no medications, including antilipidemic or antidiabetic agents. The donors provided written informed consent, and the study protocol was approved by the Institutional Review Board of Pusan National University Hospital (IRB number 2013-8).
Knife biopsies of adipose tissue were immediately placed in minimum essential medium-alpha (MEM α, Life Technologies, Carlsbad, CA, USA) supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin (Life Technologies). Samples were transported to the laboratory and processed within 30 minutes of excision. Using a sterile technique, the tissue was finely minced and digested with 0.075% type I collagenase (Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes at 37℃, with vigorous shaking. Then, 25 mL of MEM α containing 10% fetal bovine serum (FBS) were added to neutralize the collagenase, and the suspension was centrifuged at 3,000 rpm for 10 minutes. Next, samples were filtered through a 70µm nylon cell strainer (BD Biosciences, San Diego, CA, USA), washed with phosphate-buffered saline (PBS), and centrifuged at 1,600 rpm for 10 minutes. The isolated ADSCs were maintained and expanded in ADSC culture medium consisting of MEM α containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37℃ in 5% CO2. This initial culture was referred to as passage zero. The medium was replaced twice per week. When the monolayer of adherent cells reached 80% to 90% confluence, cells were trypsinized using 0.25% trypsin-EDTA (Life Technologies) and subcultured to passage three.
Flow cytometry was used to characterize the surface marker expression of passage three ADSCs. Briefly, the medium was aspirated and the cell layer was washed with PBS before incubation with 1-mL 0.25% trypsin-EDTA for 3 minutes. Monoclonal antibodies against CD90, CD44, CD73, CD105, CD31, CD19, CD11b, and HLA-DR were used (all from BD Biosciences). Cells were analyzed using a FACSAria flow cytometer (BD Biosciences). Analysis was performed on 10,000 cells per sample and unstained cell samples were used to compensate for background autofluorescence levels.
For osteogenic induction, ADSCs were seeded on six-well plates at 3×105 cells/well. Next, the medium was replaced with osteogenic medium consisting of MEM α supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, L-glutamine, 0.1 µM dexamethasone, 50 µM ascorbate-2-phosphate, and 10 mM β-glycerophosphate (Sigma-Aldrich). The medium was changed twice weekly.
On day 21 of osteogenic induction, the medium was removed and cells were washed with PBS. Cells were fixed with 70% iced ethanol for 15 minutes at 4℃ and washed with distilled water. Alizarin red S staining solution was prepared by dissolving 1 g of Alizarin red S (Sigma-Aldrich) in 100 mL of distilled water, mixing, and adjusting the pH to 4.12 with 0.1% NH4OH. Images of Alizarin red S-stained cells were captured with a DS-U2 digital sight camera (Nikon, Tokyo, Japan).
Total RNA was extracted using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Each sample, containing 2 µg of RNA, was heated at 65℃ for 15 minutes, before addition of reverse transcriptase. cDNA was prepared through incubation at 50℃ for 60 minutes using the DiaStar RT Kit (SolGent, Seoul, Korea), and real-time polymerase chain reaction (PCR) was performed using a LightCycler instrument (Roche Applied Science, Indianapolis, IN, USA). LightCycler DNA Master SYBR-Green I (Roche Applied Science), cDNA template, primer pairs, and 25 mM MgCl2 were added to microcapillary tubes to give a final volume of 20 µL. PCR was conducted for 40 to 50 cycles, each consisting of predenaturation at 95℃ for 10 seconds, 5 seconds at a specific annealing temperature, and primer extension at 72℃ for 20 seconds. The expression level of the target gene was normalized to the β-actin expression level. Melting curves were visually inspected to confirm the specificity of product detection. The primer sequences were as follows: GLP-1r, TCAAGGTCAACGGCTTATTAG (forward) and TAACGTGTCCCTAGATGAACC (reverse); osteocalcin (OC), AGCAAAGGTGCAGCCTTTGT (forward) and GCGCCTGGGTCTCTTCACT (reverse); and alkaline phosphatase (ALP), CCCCCGTGGCAACTCTATCT (forward) and GATGGCAGTGAAGGGCTTCTT (reverse).
Cells were isolated using PRO-PREPTM Protein Extraction Solution (iNtRON Biotechnology, Seoul, Korea). Protein concentrations were determined using the BCA protein assay kit (iNtRON). GLP-1R protein was separated by 10% SDS-PAGE and electroblotted onto a Hybond-ECL nitrocellulose membrane (Amersham Biosciences, Little Chalfont, UK). The membrane was blocked with 5% skim milk and incubated with GLP-1R antibody (1:1,000 dilution; Abcam, Cambridge, UK), and subsequently with horseradish peroxidase-conjugated rabbit anti-mouse IgG (Cell Signaling Technology, Danvers, MA, USA). Immunoreactive bands were detected using the West-One Western Blot Detection System (iNtRON).
All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). For all variables, descriptive statistics, including the mean and standard deviation, were determined for each day. Relative GLP-1R gene expression levels were compared by repeated measures one-way analysis of variance to test for time-dependency. P<0.05 was considered to indicate statistical significance.
ADSCs exhibited fibroblast-like morphology and adhered to plastic (Fig. 1A). To confirm their multipotent differentiation potential, specific surface antigen expression was assessed according to the minimal criteria established by the International Society for Cellular Therapy [12]. The cells showed positive expression (≥95%) of mesenchymal stem cell (MSC)-specific antigen markers, including CD90, CD73, and CD105. Conversely, expression of CD11b (a hematopoietic cell marker prominently expressed on monocytes and macrophages), CD19 (a B cell marker), CD31 (a hematopoietic cell marker expressed on the surfaces of platelets, monocytes, and neutrophils), CD34 (a marker for primitive hematopoietic progenitors and endothelial cells), and HLA-DR was not detected (Fig. 1B).
Osteogenic differentiation was confirmed by cytochemical staining and gene expression analysis. After 21 days of osteogenic induction, positive Alizarin red S staining consistent with matrix calcification was observed (Fig. 2A). ALP is a commonly used marker for early osteogenic differentiation. In our study, ALP was notably upregulated at the 21 days (Fig. 2B). Expression of the late osteogenic marker OC is known to be upregulated immediately prior to mineralization, and we observed significant increases in OC mRNA levels by day 21 of differentiation (Fig. 2C).
Up-regulation of GLP1-R protein expression during the osteoblastic developmental sequence was confirmed by Western blot data for day 21 (Fig. 3A). Real-time PCR was used to assess the time-course of GLP-1R mRNA expression during osteogenic differentiation. For each time-point (0, 7, 14, and 21 days), the GLP-1R mRNA level of each sample was normalized to that of β-actin. The mean GLP-1R activity of ADSCs increased significantly in a time-dependent manner that was related to the degree of differentiation (P=0.013) (Fig. 3B).
In this study, we demonstrated that GLP-1R is expressed on ADSC-derived osteogenic cells, and that the level of expression increases in a time-dependent manner during differentiation. GLP-1 has been consistently reported to have a beneficial effect on bone in rodents [2,3]. Also, it is reported to have no adverse effects on bone, despite inducing weight loss in humans [13]. A GLP-1R agonist has been reported to prevent osteopenia by promoting bone formation and by suppressing bone resorption in aged, ovarectomised rats [14]. Furthermore, a GLP-1 agonist increased bone mineral density in type 2 diabetic rats [15]. The mechanism underlying this phenomenon is not clear. One possible explanation is that GLP-1Rs expressed on thyroid C cells promote calcitonin secretion, which inhibits bone resorption; however, GLP-1R was not detected in osteoblasts [16]. A second possibility is the presence of a functional receptor independent of the cAMP-linked GLP-1R. A final possibility is the existence of a GLP-1R.
The downstream effect of GLP-1 is mediated by a G-protein-coupled receptor that is expressed in various tissues, such as the pancreas, stomach, and vascular system [17]. Expression of GLP-1R by osteoblasts, however, has not been confirmed. GLP-1R has been reported to be expressed on thyroid C cells, which affect bone resorption by promoting calcitonin secretion, but the study did not detect GLP-1R expression in osteoblasts [16]. Conversely, expression of GLP-1R was reported in MC3T3-E1 cells, a well-known mouse osteoblastic cell line [4]. The authors suggested that this result indicates that, like other classic receptors, GLP-1R ha nd temperature-dependent. Another study reported that GLP-1R is expressed by osteoblastic cell lines, but that the expression level is cell line-dependent, perhaps reflecting different stages of osteoblast differentiation [5]. In that study, GLP-1R was expressed by young osteoblasts but not by mature osteoblasts derived from the Saos-2 osteosarcoma cell line. It was suggested that GLP-1R is expressed by osteoblasts, but that the expression decreases with maturation. However, GLP-1R expression was recently reported in osteocytes, which are derived from osteoblasts [15]. In addition, GLP-1R expression may differ among species [18,19].
We also evaluated the levels of OC and ALP in our ADSC samples. One of the most common methods used to examine mineralization is Alizarin red S staining, which indicates extracellular calcification. Alizarin red staining alone, however, may not be sufficient to confirm osteogenic differentiation [20,21]. ALP is a well-known indicator of the early stage of osteogenic differentiation of MSCs, and expression of ALP increases with osteoblast maturation [22]. In this study, ALP levels increased in a time-dependent manner during osteogenic differentiation of ADSCs. OC is a noncollagenous protein found in bone and dentin and is generally considered to be a relatively late-stage marker for the period of osteoblastic differentiation immediately prior to mineralization [23,24]. In our study, expression of both ALP and OC was elevated on day 21 of differentiation, confirming that osteogenic differentiation of ADSCs had occurred.
In summary, we demonstrated GLP-1R expression during osteogenic differentiation of ADSCs. Even though it is not yet clear whether GLP-1 has a role in bone metabolism, our results suggest that it may induce osteogenic differentiation in bone tissue. Further studies are needed to explore this possibility.
Figures and Tables
Fig. 1
Adipose-derived stem cells (ADSCs). (A) ADSCs exhibited fibroblast-like morphology and adhered to plastic, as assessed by light microscopy (×40) at passage three. (B) Flow cytometry using mesenchyme-specific antigen markers showed that ADSCs express CD73, CD90, and CD105, but not CD11b, CD19, CD31, and HLA-DR.

Fig. 2
Osteogenic differentiation of adipose-derived stem cells. (A) Calcification, detected by Alizarin red staining and indicative of mineralization, was assessed by (Aa) gross appearance and (Ab) light microscopy (×40) at days 0, 7, 14, and 21. Real-time polymerase chain reaction showed increased expression of (B) alkaline phosphatase (ALP) and (C) osteocalcin mRNA at day 21. Data are presented as means±standard deviation.
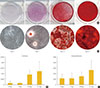
Fig. 3
Expression of glucagon-like peptide 1 receptor (GLP-1R) during osteogenic differentiation of adipose-derived stem cells (ADSCs). (A) Western blotting for GLP-1R in differentiating ADSCs at days 0, 7, 14, and 21 after differentiation. Data are presented as means±standard deviation. (B) The expression level of GLP-1R mRNA increased significantly in a time-dependent manner during osteogenic differentiation (P=0.013). GAPDH, glyceraldehyde 3-phosphate dehydrogenase. aP<0.05.
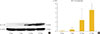
ACKNOWLEDGMENTS
This study was supported by a Biomedical Research Institute Grant (2013-10) from Pusan National University Hospital.
References
1. Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003; 17:161–171.
2. Nuche-Berenguer B, Lozano D, Gutierrez-Rojas I, Moreno P, Marinoso ML, Esbrit P, Villanueva-Penacarrillo ML. GLP-1 and exendin-4 can reverse hyperlipidic-related osteopenia. J Endocrinol. 2011; 209:203–210.
3. Nuche-Berenguer B, Moreno P, Esbrit P, Dapia S, Caeiro JR, Cancelas J, Haro-Mora JJ, Villanueva-Penacarrillo ML. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int. 2009; 84:453–461.
4. Nuche-Berenguer B, Portal-Nunez S, Moreno P, Gonzalez N, Acitores A, Lopez-Herradon A, Esbrit P, Valverde I, Villanueva-Penacarrillo ML. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010; 225:585–592.
5. Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011; 11:12.
6. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.
7. Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005; 87:125–128.
8. Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003; 58:137–160.
9. Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett. 2011; 16:236–257.
10. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006; 99:1285–1297.
11. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004; 22:560–567.
12. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.
13. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006; 8:436–447.
14. Ma X, Meng J, Jia M, Bi L, Zhou Y, Wang Y, Hu J, He G, Luo X. Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J Bone Miner Res. 2013; 28:1641–1652.
15. Kim JY, Lee SK, Jo KJ, Song DY, Lim DM, Park KY, Bonewald LF, Kim BJ. Exendin-4 increases bone mineral density in type 2 diabetic OLETF rats potentially through the down-regulation of SOST/sclerostin in osteocytes. Life Sci. 2013; 92:533–540.
16. Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008; 149:574–579.
17. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007; 87:1409–1439.
18. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010; 151:1473–1486.
19. Hirsch PF, Baruch H. Is calcitonin an important physiological substance? Endocrine. 2003; 21:201–208.
20. Boyan BD, Schwartz Z, Boskey AL. The importance of mineral in bone and mineral research. Bone. 2000; 27:341–342.
21. Declercq HA, Verbeeck RM, De Ridder LI, Schacht EH, Cornelissen MJ. Calcification as an indicator of osteoinductive capacity of biomaterials in osteoblastic cell cultures. Biomaterials. 2005; 26:4964–4974.
22. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995; 17:2 Suppl. 77S–83S.
23. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990; 143:213–221.
24. Koshihara Y, Kawamura M, Endo S, Tsutsumi C, Kodama H, Oda H, Higaki S. Establishment of human osteoblastic cells derived from periosteum in culture. In Vitro Cell Dev Biol. 1989; 25:37–43.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download