Abstract
Since the adoption of the Da Vinci robotic system for remote access thyroid surgery, robotic thyroidectomy (RT) has become a popular surgical option for patients who want to avoid neck scars. Surgeons in South Korea pioneered this surgical technique and have reported successful outcomes. Although many studies have reported that RT is a feasible and safe therapeutic alternative, concerns over the surgical and oncological safety of RT remain. This article reviews the advantages and disadvantages of RT and compares the surgical safety and oncological completeness of RT with conventional open thyroidectomy.
Thyroid carcinoma is the most common endocrine malignancy [1]. Although the treatment of choice has been conventional open thyroidectomy (OT), this method inevitably results in neck scars due to the anatomical location of the thyroid gland. To avoid cosmetically undesirable outcomes, various remote approaches have been utilized for patients with low risk of recurrence, the two most common techniques being the transaxillary approach (TAA) and bilateral axillo-breast approach (BABA).
In the TAA, the patient's lesion-side arm is raised over the head, and a 5- to 6-cm vertical skin incision is made in the axilla. Surgical instruments are inserted through a subcutaneous skin flap running from the axilla to the anterior neck, with surgery continuing from the lateral side (Fig. 1) [2]. In contrast, BABA uses two 0.8-cm-sized axillar incisions and two circumareolar incisions, measuring 0.8-cm on the left and 1.2-cm on the right side. The 1.2-cm circumareolar incision is used as a camera port and can be moved to the left side if the surgeon prefers [3]. Both the TAA and BABA techniques were originally developed for endoscopic surgery, but they have been generally replaced by robotic surgery since the adoption of the Da Vinci robotic system in 2007. Robotic surgery has advantages over endoscopic surgery, including superior field of view, ergonomic improvement, and learning curve [4,5]. The merits of the robotic system, including high-definition three-dimensional imaging, high degree of freedom of motion, and a tremor filtering system, have increased the popularity of robotic thyroidectomy (RT).
Despite many studies reporting that RT is effective and safe in patients with thyroid cancer, not all thyroid surgeons advocate RT. RT has several disadvantages, including being costly, technically demanding, and requiring advanced training [6]. To date, however, no randomized controlled study has compared RT with OT. This review of previously published studies compares the outcomes of RT with those of OT.
The most important motivation for patients to choose RT is the absence of neck scarring. For example, a prospective questionnaire study of 41 patients who underwent RT and 43 patients who underwent OT evaluated cosmetic satisfaction 3 months postoperatively [7]. In a second study, cosmetic satisfaction was assessed after 1 day, 1 week, and 1 or 3 months in 75 patients who underwent RT and 226 who underwent OT [8]. In both studies, cosmetic satisfaction was greater in the RT than in the OT group at all recorded time points. Cosmetic satisfaction in patients who underwent modified radical neck dissection (MRND) was also higher in the RT than in the OT group [9]. Thus, the cosmetic benefits of RT seem to be established.
Since RT requires the formation of a larger skin flap than OT, concerns have arisen that postoperative neck and chest pain may be greater after RT. Contrary to this assumption, levels of pain were similar after RT and OT. For example, a questionnaire evaluation of patients 24 hours after thyroidectomy found no significant difference in pain after RT and OT [7]. Another study evaluating pain on a visual analogue scale 30 minutes, 4 hours, and 1, 2, 3, and 10 days after RT or OT found no difference in analgesic use, with the pain score being lower in the RT group than in the OT group at 1 and 2 days after surgery [10]. An evaluation of neck and chest pain found that neck pain was similar in the RT and OT groups, although anterior chest pain was significantly greater in the RT group [8]. However, chest pain score in the RT group was lower than neck pain score in both groups.
Table 1 shows the incidence of transient and permanent recurrent laryngeal nerve injury (RLNI) who underwent robotic or OT. Most of the studies defined transient RLNI as hoarseness or vocal cord palsy persisting less than 6 months. Six studies comparing RT with OT found similar rates of transient/permanent RLNI [7,8,11,12,13,14]. The incidence of transient RLNI in patients who underwent RT varied from 0% to 20%, with most studies reporting a rate less than 15%. Permanent RLNI was observed in only 0.2% to 0.3% of the patients [4,15,16,17,18], a rate comparable to that of open surgery [19].
Although the definition of hypoparathyroidism differs among studies, it is generally based on parathyroid hormone, calcium level, or on hypocalcemic symptoms (Table 2). In most studies, a diagnosis of permanent hypoparathyroidism required the condition to last more than 6 months. In six studies comparing RT and OT [7,8,11,13,14,17], the incidence of permanent hypoparathyroidism was similar in the two groups, although one study [17] reported a higher rate of transient hypocalcemia in the RT group.
Other rare complications are described in Table 3. Unique complications associated only with RT included brachial injury and skin flap perforation, although their incidence was only 0.1% in the study with the largest patient population [18]. Bleeding is considered a major complication of both RT and OT, but hematomas requiring reoperation were uncommon in the RT group [4,8,12,18].
Since RT requires the formation of a larger skin flap than OT, RT may be accompanied by sensory changes in the corresponding skin area. For example, an evaluation of cutaneous light-pressure thresholds in patients who underwent BABA RT found sensory changes in the anterior chest area; however, these became normalized 3 months after surgery [20]. Similar results were observed in patients who undewent TAA RT. Two studies prospectively comparing sensory changes in patients who underwent TAA RT and OT found that sensory changes in the anterior neck area were similar or more common in the OT group, whereas sensory changes in the anterior chest were more common in the RT group. Anterior chest discomfort in the RT group became comparable with that in the OT group 1.5 years after surgery [21]. Sensory changes, therefore, were considered fairly minor.
Among the clinical parameters used to evaluate surgical completeness after thyroidectomy are the number of retrieved lymph nodes, stimulated thyroglobulin (sTg) concentration, and raidioactive iodine uptake (RAI) on whole body scans (WBS).
Since papillary thyroid carcinomas metastasize via the surrounding lymph nodes, central lymph node dissection is routinely performed in most centers in order to assure oncological safety. In most studies, the numbers of retrieved central lymph nodes were comparable during RT and OT (Table 4). In two studies [8,14], however, the absolute number of retrieved lymph nodes was lower in patients undergoing RT than OT (4.4±2.4 and 8.7±5.1, respectively), although the absolute numbers of the former were similar to those in other studies.
sTg level is measured before RAI treatment after elevating thyroid stimulating hormone (TSH) either by thyroid hormone withdrawal or by injection of recombinant human TSH (Thyrogen, Genzyme, Cambridge, MA, USA). Elevated sTg after total thyroidectomy suggests the presence of remnant thyroid tissue; therefore, low sTg is a good indicator of complete thyroid removal. Table 4 shows the results of sTg at the first RAI treatment after RT or OT. While four studies (three BABA, one TAA) reported similar sTg levels in these two groups [11,14,22,23], the other two studies (both TAA) reported that sTg was significantly higher in the RT than in the OT group [8,17]. However, in these two studies, sTg levels turned out to be similar after stage subclassification [17] or after the first RAI treatment [8].
Remnant thyroid tissue can also be measured by thyroid uptake count ratio on WBS. For example, in one study, RAI uptake in the thyroid bed was higher in the RT than in the OT group when assessed by WBS [23]. The higher uptake in the RT group may have been due to the characteristics of TAA, in which removal of the contralateral thyroid gland is made difficult by the unilateral approach. However, RAI uptake was similar in the RT and OT groups at the second WBS after the first RAI treatment. In contrast, when BABA RT and OT were compared after propensity score matching to minimize selection bias, RAI uptake was similar at the first WBS in the two groups [22].
Graves disease has been regarded as a contraindication for endoscopic thyroidectomy or RT because bleeding control is hampered by the narrow operative view, hypervascularity, and the large-sized thyroid gland. However, recent technical advances and accumulated experience have enabled RT in patients with Graves disease. Kwon et al. [24] reported successful RT in 30 patients with Graves disease, with a tolerable complication rate. In the study, one patient experienced permanent hypoparathyroidism, but none had major complications such as bleeding, open conversion, or permanent RLNI. The other retrospective study comparing RT and OT in Graves' disease patients [25], in which those with smaller-sized thyroid underwent RT, found no significant between-group differences in operation time, blood loss, or complication rate. RT might be performed safely in selected Graves' disease patients.
Robotic surgery has also been utilized for MRND [26]. Since extensive incision scars are inevitable in patients undergoing traditional MRND, robotic MRND may be a good alternative to avoid scarring. Both TAA and BABA have shown outcomes similar to open conventional MRND. For example, a similar number of lateral lymph nodes was retrieved following BABA robotic and open MRND, without causing hypocalcemia, RLNI, or bleeding [27]. In addition, a comparison of patients undergoing TAA or open MRND reported similar complication, surgical completeness, and recurrence rates [9].
RT offers better cosmetic satisfaction to patients than OT, and RT is as safe and effective as OT in thyroid cancer patients, with similar complication rates. RT also showed similar oncologic safety as OT, as assessed by lymph node harvest, complete removal of thyroid tissue, and RAI success rates. RT is a good alternative surgical modality for patients who wish to avoid neck scars.
Figures and Tables
Fig. 1
Patient position and flap dissection area (red color) of transaxillary approach (A) and bilateral axillo-breast approach (B).
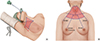
Table 4
Surgical Completeness of Robotic Thyroidectomy

Values are expressed as number (%).
TT, total thyroidectomy; RT, robotic thyroidectomy; OT, open thyroidectomy; TAA, transaxillary approach; BABS, bilateral axillo-breast approach; NA, not available.
aPercentage of the patients who had <2 ng/mL of stimulated thyroglobulin at the first radioactive iodine treatment.
References
1. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005; 12:245–262.
2. Kang SW, Jeong JJ, Nam KH, Chang HS, Chung WY, Park CS. Robot-assisted endoscopic thyroidectomy for thyroid malignancies using a gasless transaxillary approach. J Am Coll Surg. 2009; 209:e1–e7.
3. Lee KE, Choi JY, Youn YK. Bilateral axillo-breast approach robotic thyroidectomy. Surg Laparosc Endosc Percutan Tech. 2011; 21:230–236.
4. Lee KE, Kim E, Koo do H, Choi JY, Kim KH, Youn YK. Robotic thyroidectomy by bilateral axillo-breast approach: review of 1,026 cases and surgical completeness. Surg Endosc. 2013; 27:2955–2962.
5. Kang SW, Jeong JJ, Yun JS, Sung TY, Lee SC, Lee YS, Nam KH, Chang HS, Chung WY, Park CS. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc. 2009; 23:2399–2406.
6. Inabnet WB 3rd. Robotic thyroidectomy: must we drive a luxury sedan to arrive at our destination safely? Thyroid. 2012; 22:988–990.
7. Lee J, Nah KY, Kim RM, Ahn YH, Soh EY, Chung WY. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc. 2010; 24:3186–3194.
8. Tae K, Ji YB, Cho SH, Lee SH, Kim DS, Kim TW. Early surgical outcomes of robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach for papillary thyroid carcinoma: 2 years' experience. Head Neck. 2012; 34:617–625.
9. Lee J, Kwon IS, Bae EH, Chung WY. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab. 2013; 98:2701–2708.
10. Ryu HR, Lee J, Park JH, Kang SW, Jeong JJ, Hong JY, Chung WY. A comparison of postoperative pain after conventional open thyroidectomy and transaxillary single-incision robotic thyroidectomy: a prospective study. Ann Surg Oncol. 2013; 20:2279–2284.
11. Kim WW, Kim JS, Hur SM, Kim SH, Lee SK, Choi JH, Kim S, Lee JE, Kim JH, Nam SJ, Yang JH, Choe JH. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World J Surg. 2011; 35:779–784.
12. Landry CS, Grubbs EG, Warneke CL, Ormond M, Chua C, Lee JE, Perrier ND. Robot-assisted transaxillary thyroid surgery in the United States: is it comparable to open thyroid lobectomy? Ann Surg Oncol. 2012; 19:1269–1274.
13. Noureldine SI, Jackson NR, Tufano RP, Kandil E. A comparative North American experience of robotic thyroidectomy in a thyroid cancer population. Langenbecks Arch Surg. 2013; 398:1069–1074.
14. Kim BS, Kang KH, Kang H, Park SJ. Central neck dissection using a bilateral axillo-breast approach for robotic thyroidectomy: comparison with conventional open procedure after propensity score matching. Surg Laparosc Endosc Percutan Tech. 2014; 24:67–72.
15. Yoo H, Chae BJ, Park HS, Kim KH, Kim SH, Song BJ, Jung SS, Bae JS. Comparison of surgical outcomes between endoscopic and robotic thyroidectomy. J Surg Oncol. 2012; 105:705–708.
16. Kim HY, d'Ajello F, Woo SU, Son GS, Lee JB, Bae JW. Robotic thyroid surgery using bilateral axillo-breast approach: personal initial experience over two years. Minerva Chir. 2012; 67:39–48.
17. Yi O, Yoon JH, Lee YM, Sung TY, Chung KW, Kim TY, Kim WB, Shong YK, Ryu JS, Hong SJ. Technical and oncologic safety of robotic thyroid surgery. Ann Surg Oncol. 2013; 20:1927–1933.
18. Ban EJ, Yoo JY, Kim WW, Son HY, Park S, Lee SH, Lee CR, Kang SW, Jeong JJ, Nam KH, Chung WY, Park CS. Surgical complications after robotic thyroidectomy for thyroid carcinoma: a single center experience with 3,000 patients. Surg Endosc. 2014; Epub 2014 Mar 20. DOI: http://dx.doi.org/10.1007/s00464-014-3502-1.
19. Barczynski M, Konturek A, Pragacz K, Papier A, Stopa M, Nowak W. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg. 2014; 38:599–606.
20. Kim SJ, Lee KE, Myong JP, Koo do H, Lee J, Youn YK. Prospective study of sensation in anterior chest areas before and after a bilateral axillo-breast approach for endoscopic/robotic thyroid surgery. World J Surg. 2013; 37:1147–1153.
21. Song CM, Ji YB, Bang HS, Park CW, Kim H, Tae K. Long-term sensory disturbance and discomfort after robotic thyroidectomy. World J Surg. 2014; 38:1743–1748.
22. Lee KE, Koo do H, Im HJ, Park SK, Choi JY, Paeng JC, Chung JK, Oh SK, Youn YK. Surgical completeness of bilateral axillo-breast approach robotic thyroidectomy: comparison with conventional open thyroidectomy after propensity score matching. Surgery. 2011; 150:1266–1274.
23. Lee S, Lee CR, Lee SC, Park S, Kim HY, Son H, Kang SW, Jeong JJ, Nam KH, Chung WY, Park CS, Cho A. Surgical completeness of robotic thyroidectomy: a prospective comparison with conventional open thyroidectomy in papillary thyroid carcinoma patients. Surg Endosc. 2014; 28:1068–1075.
24. Kwon H, Koo do H, Choi JY, Kim E, Lee KE, Youn YK. Bilateral axillo-breast approach robotic thyroidectomy for Graves' disease: an initial experience in a single institute. World J Surg. 2013; 37:1576–1581.
25. Noureldine SI, Yao L, Wavekar RR, Mohamed S, Kandil E. Thyroidectomy for Graves' disease: a feasibility study of the robotic transaxillary approach. ORL J Otorhinolaryngol Relat Spec. 2013; 75:350–356.
26. Kang SW, Lee SH, Ryu HR, Lee KY, Jeong JJ, Nam KH, Chung WY, Park CS. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery. 2010; 148:1214–1221.
27. Kim BS, Kang KH, Park SJ. Robotic modified radical neck dissection by bilateral axillary breast approach for papillary thyroid carcinoma with lateral neck metastasis. Head Neck. 2013; Epub 2013 Nov 8. DOI: http://dx.doi.org/10.1002/hed.23545.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download