Abstract
Papillary thyroid carcinoma (PTC) is a common affliction of the thyroid gland, accounting for 70% to 80% of all thyroid cancers, whereas mucosa-associated lymphoid tissue (MALT) lymphoma of the thyroid gland is uncommon. The simultaneous occurrence of both malignancies is extremely rare. We report the case of a patient with both PTC and MALT lymphoma in the setting of Hashimoto thyroiditis. An 81-year-old female patient was first admitted with goiter and hoarseness, which was attributed to an ultrasonographic thyroid nodule. Subsequent fine-needle aspirate, interpreted as suspicious of papillary thyroid cancer, prompted total thyroidectomy. MALT lymphoma was an incidental postsurgical finding, coexisting with PTC in the setting of Hashimoto thyroiditis. Although the development of MALT lymphoma is very rare, patients with longstanding Hashimoto thyroiditis should undergo careful surveillance for both malignancies.
Papillary carcinoma is the most prevalent type of thyroid cancer [1,2], whereas primary lymphoma of the thyroid gland is distinctly uncommon, accounting for only 0.5% to 5% of all thyroid malignancies and 2% to 7% of all extranodal lymphomas [3,4]. As an autoimmune disease, Hashimoto thyroiditis (HT) is characterized by widespread lymphocytic infiltration, fibrosis, and parenchymal atrophy. It is the most common cause of hypothyroidism.
The contribution of HT to papillary thyroid carcinoma (PTC) remains controversial, but lymphoma of the thyroid is viewed as a consequence of long-standing autoimmune thyroiditis. In prior studies, the reported incidence of HT in patients with primary thyroid lymphomas ranged from 27% to 100% [3]. Furthermore, previous reports indicate that the likelihood of developing thyroidal lymphoma is 40 to 80 times greater in patients with chronic thyroiditis, relative to the general population [5].
Although PTC and mucosa-associated lymphoid tissue (MALT) lymphoma have each been separately reported in conjunction with HT, the coexistence of PTC and MALT lymphoma is extremely rare. To our knowledge, only two such occurrences have been documented in the literature [6,7].
Herein we report an 81-year-old female patient who underwent total thyroidectomy for probable PTC. PTC was ultimately confirmed, and MALT lymphoma of the thyroid was also incidentally discovered.
An 81-year-old female patient was admitted to a local hospital for weight loss, goiter, and hoarseness. The goiter and hoarseness had been present for about 10 years; the weight loss was 10 kg over the prior 6 months. However, there were no symptoms of fever or night sweats. She had a history of hypertension, but had no family history of cancer, and had no history of thyroid disease or radiation exposure. On physical examination, the thyroid was firm, fixed, and diffusely enlarged; however, there was no pain or tenderness. The thyroid gland measured approximately 10 cm in width and 12 cm in length. Other than the goiter, physical examination was unremarkable. Laboratory tests were as follows: white blood cell count, 6,760/µL; hemoglobin, 13.4 g/dL; platelet count, 207,000/µL; total protein, 9.5 g/dL; albumin, 3.7 g/dL; aspartate aminotransferase, 25 IU/L; alanine aminotransferase, 16 IU/L; lactate dehydrogenase, 257 IU/L (normal range, 135 to 214). Thyroid function tests: free T4, 0.79 ng/dL (normal range, 0.75 to 2.00); T3, 99.5 ng/dL (normal range, 79.8 to 200); thyroid stimulating hormone (TSH), 2.55 µU/mL (normal range, 0.3 to 5.0); and antithyroglobulin antibody level, 14.75 U/mL (normal range, 0 to 60) were within normal limits, but antithyroid peroxidase antibodies were elevated (165.22 U/mL; normal range, 1 to 60).
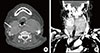
Initial ultrasonography revealed diffuse enlargement of the thyroid gland, with heterogeneous background parenchyma. Transverse imaging revealed an ill-defined hypoechoic nodule measuring 3.7×3 cm in the right thyroid gland. The left thyroid gland and isthmus showed a diffuse goiter with ill-defined multiple patchy hypoechogenicities and heterogenecity (Fig. 1). Fine needle aspiration revealed the nodule to be suspicious for PTC. Thereafter, the patient was admitted to our hospital for surgery. Preoperative computed tomography (CT) of the neck revealed a diffusely enlarged thyroid gland with nodules in both lobes (Fig. 2).
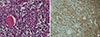
A total thyroidectomy and right and left central neck node dissection was performed. Gross and microscopic examination disclosed a 3.7×3.0 cm papillary carcinoma in the right thyroid gland (Fig. 3). There was minimal extrathyroidal extension, all surgical resection margins were negative and no metastatic lymph nodes were found (pathologic TNM staging, T3N0Mx, stage III). Background thyroid tissue showed diffuse lymphocyte infiltration, the presence of Hürthle cells, small follicles with scanty colloid, and germinal center formation, which are all characteristics of HT. The left thyroid gland weighed 116 g and was measured 9.6×6.9×2.9 cm; microscopic histopathology revealed a nodular proliferation of small B cells, with a lymphoepithelial lesion suggestive of MALT lymphoma in the left thyroid gland. Much of the normal thyroid architecture was replaced by dense, diffuse infiltrates of atypical small B lymphocytes, showing strong CD20- and lambda light chain-immunopositivity, and an absence of κ light chain (Fig. 4). IgH gene rearrangement assay confirmed the diagnosis of MALT lymphoma.
Bone marrow examination is necessary in order to set the stage of lymphoma. However, the patient refused invasive evaluation due to her age. Postoperative neck CT and 18F-fluorodeoxyglucose positron emission tomography of the neck performed within 1 month of surgery demonstrated no persistence or recurrence of either neoplasm. There was no evidence of lymph node enlargement in the neck, mediastinum, or abdomen. Although a bone marrow biopsy was not performed, MALT lymphoma was considered to be confined to the thyroid gland, corresponding to stage IE according to Ann Arbor classification.
The patient is currently taking levothyroxine as a sole postsurgical therapy. Based on age and health status, radioiodine treatment was not administered. Symptoms of hoarseness and weight loss improved after surgery. One year after surgery, a thyroid function test was performed and antithyroglobulin antibody levels and thyroglobulin levels were measured (free T4, 1.61 ng/dL; T3, 122.1 ng/dL; TSH, 0.00 µU/mL; antithyroglobulin antibody, 11.02 U/mL; thyroiglobulin, 0.227 ng/mL). Neither tumor has recurred during the 1-year follow-up period.
In Korea, widespread use of ultrasonography is increasing the detection of microscopic PTC. The prevalence of PTC has thus increased, while the prevalence of lymphoma among all thyroid cancers has declined [3]. MALT lymphoma often involves the stomach, but any mucosal site may be affected, including the intestine, salivary gland, orbit, thyroid gland, lung, or liver. Development of MALT lymphoma has been linked to chronic inflammation and autoimmunity, hence it is associated with HT in the thyroid gland. Despite the fact that thyroidal MALT lymphoma is actually quite rare, primary lymphoma deserves due diagnostic consideration in any patient with HT who develops a neck mass.
Diffuse large B-cell lymphoma (DLBCL) and MALT lymphoma are the most common subtypes involved in primary lymphoma of the thyroid. However, patient prognosis is generally better in MALT lymphoma than in DLBCL [8,9]. Treatment of thyroid lymphoma is dependent on stage and subtype. Localized MALT lymphoma of thyroid may be treated with surgical resection [8]. If the disease is localized, overall and disease-free survival estimates after total thyroidectomy for MALT lymphoma are 100% at 5 years [4,9]. Tsang et al. [10] reviewed 103 cases of localized (stage IE or IIE) extranodal MALT lymphoma. The overall 5-year survival was 98%, with a disease-free survival of 77%, in 85 patients with extranodal MALT lymphoma treated with radiation therapy alone. A total of 13 patients had thyroid lymphoma, and they had a 100% disease control rate [10]. In our patient, MALT lymphoma was confined to the thyroid gland, so surgical resection alone seemed adequate. Thyroid lymphoma usually involves either the right or left lobe, or both lobes, of the thyroid, and may present as a solitary nodule [11]. However, MALT lymphoma in this case was not a solitary nodule, but involved both lobes of the thyroid.
Papillary carcinoma has an excellent prognosis (20-year survival rate, >90%), in addition to being the most common variant of thyroid cancer. In general, postoperative management of thyroid cancer is multifactorial, considering the extent of disease at surgery, histologic type and grade of tumors, age of the patient, and assigned risk group. Adjunctive radioiodine treatment was not recommended in this instance, given the patient's age and general medical condition.
In the event that PTC and MALT lymphoma do coexist, treatment strategies may vary based on pathologic findings, tumor size and stage, and clinical symptoms. Therapy should be individualized, aimed at whichever tumor is more aggressive, but ideal management entails optimal treatment of both diseases.
Herein, we report a case of PTC and MALT lymphoma coexisted in the setting of HT. Total thyroidectomy proved effective as the sole therapeutic intervention.
Figures and Tables
Fig. 1
(A) Thyroid ultrasonography shows an ill-defined hypoechogenic mass measuring 3.7×3.0 cm with microcalcifications in the right thyroid gland. (B) Left thyroid gland and isthmus show diffuse goiter with ill-defined multiple patchy hypoechogenicities and a heterogenicity.

Fig. 2
(A) Precontrast axial computed tomography (CT) scan shows a low density nodule (arrow) in the upper portion of the right thyroid gland. (B) Contrast-enhanced coronal CT scan shows a large, inhomogeneous enhancement of the left thyroid gland, displacing the trachea to the right, and a hyperintense nodule in the upper portion of the right thyroid gland (arrow).
References
1. Busnardo B, De Vido D. The epidemiology and etiology of differentiated thyroid carcinoma. Biomed Pharmacother. 2000; 54:322–326.
2. Hakala T, Kellokumpu-Lehtinen P, Kholova I, Holli K, Huhtala H, Sand J. Rising incidence of small size papillary thyroid cancers with no change in disease-specific survival in finnish thyroid cancer patients. Scand J Surg. 2012; 101:301–306.
3. Hwang YC, Kim TY, Kim WB, Shong YK, Yi KH, Shong M, Jo YS, Kim WS, Chung JH. Clinical characteristics of primary thyroid lymphoma in Koreans. Endocr J. 2009; 56:399–405.
4. Derringer GA, Thompson LD, Frommelt RA, Bijwaard KE, Heffess CS, Abbondanzo SL. Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol. 2000; 24:623–639.
5. Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985; 312:601–604.
6. Cheng V, Brainard J, Nasr C. Co-occurrence of papillary thyroid carcinoma and primary lymphoma of the thyroid in a patient with long-standing Hashimoto's thyroiditis. Thyroid. 2012; 22:647–650.
7. Melo GM, Sguilar DA, Petiti CM, Eichstaedt AG, Caiado RR, Souza RA. Concomitant thyroid Malt lymphoma and papillary thyroid carcinoma. Arq Bras Endocrinol Metabol. 2010; 54:425–428.
8. Mack LA, Pasieka JL. An evidence-based approach to the treatment of thyroid lymphoma. World J Surg. 2007; 31:978–986.
9. Thieblemont C, Mayer A, Dumontet C, Barbier Y, Callet-Bauchu E, Felman P, Berger F, Ducottet X, Martin C, Salles G, Orgiazzi J, Coiffier B. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab. 2002; 87:105–111.
10. Tsang RW, Gospodarowicz MK, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Patterson BJ. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003; 21:4157–4164.
11. Kini SR. Thyroid cytopathology: an atlas and text. 1st ed. Philadelphia: Lippincott Williams & Wilkins;2008. p. 322–326.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download