Abstract
A 53-year-old woman was presented with several 0.3-0.6 cm-sized nodules within the right lobe of the thyroid. Histologic sections of the thyroid demonstrated multiple papillary microcarcinomas in the background of lymphocytic thyroiditis, with a small focus of Langerhans cell histiocytosis (LCH). Small LCH nodules were also found in the draining cervical lymph nodes. Although the association of LCH with papillary thyroid carcinoma in the thyroid has been reported, their co-existence with LCH in the draining lymph nodes is very rare.
Langerhans cell histiocytosis (LCH) is a rare disorder characterized by proliferation of abnormal clusters of differentiation 1a-positive (CD1a) dendritic cells [1]. It may cause local or systemic effect [1,2]. LCH may be associated with Hodgkin's disease, non-Hodgkin's lymphomas, carcinomas and other solid tumors [3,4]. A limited number of thyroid LCH with co-existing papillary carcinoma has been reported in the literature. Involvement of the thyroid gland can be focal or diffuse, and results in organ involvement, occasionally accompanied by nodular goiter, lymphocytic thyroiditis, or papillary carcinoma [5,6]. Moreover, LCH occurring in lymph nodes draining thyroid papillary carcinoma is extremely rare. We report a very rare case of thyroid LCH with papillary carcinoma and co-existing LCH of draining lymph nodes.
A 53-year-old woman presented with a progressively enlarging thyroid over 5 years. Laboratory studies including thyroid function tests, chemistry profile, complete blood cell count with differential, platelet count, red blood cell distribution width, and prothrombin and partial thromboplastin times were all within the normal limits. Thyroid function studies disclosed free T4 and T3 levels were within the normal limits.
Ultrasonography revealed three calcified masses measuring 0.6, 0.4, and 0.3 cm. Fine needle aspiration was performed several times but failed due to inadequate specimen.
The patient underwent right thyroid lobectomy with right lymph node dissection. Pathologic examination of the right lobe revealed multiple calcified nodules measuring up to 0.6 cm in the greatest dimension.
Microscopic examination of the nodules revealed papillary microcarcinomas (Fig. 1). The remainder of the thyroid tissue showed lymphocytic thyroiditis and a separate, incidental, 0.5 cm-sized nodule (Fig. 2A). Microscopic examination of this nodule revealed poorly circumscribed proliferation of large cells effacing the follicular architecture of the thyroid. The cells comprising the nodules were arranged in sheets and small cluster, and exhibited abundant eosinophilic cytoplasm and bean-shaped, folded nuclei admixed with eosinophils (Fig. 3). Immunohistochemical stain for S100 was strongly positive in the large cells with folded nuclei, while pancytokeratin was negative (Fig. 2B, C). Although immunohistochemical stain for CD1a and electron microscopy were not performed, the diagnosis of LCH of the thyroid gland was made based on microscopical and immunohistochemical results. Examination of the right paratracheal lymph nodes revealed incidental, small nodules measuring up to 0.2 cm in the greatest dimension, and the nodules showed clusters of cells with abundant eosinophilic cytoplasm and bean-shaped, folded nuclei admixed with eosinophils (Fig. 4A). The diagnosis of LCH in draining lymph nodes was made. Immunohistochemical stain for S100 was strongly positive in the large cells with folded nuclei (Fig. 4B).
Evidence of LCH multi-focality was not confirmed because follow-up studies, such as bone marrow aspiration and biopsy, bone scan, and computed tomography of the neck, chest, abdomen, and pelvis were not performed in this patient.
Hematoxylin-eosin-stained sections were examined. Immunohistochemical studies were conducted on formalin-fixed, paraffin-embedded, 4 µm-thick tissue sections. The primary antibodies used were murine monoclonal anti-pancytokeratin (prediluted; AE1/AE3, Dako, Glostrup, Denmark), and S-100 (prediluted; Dako). Tissue sections were deparaffinized three times in xylene for a total of 15 minutes and subsequently rehydrated. Immunostaining was performed using a Bond-max autoimmunostainer (Leica Biosystem, Melbourne, Australia) with ER1 or ER2 retrieval buffers and a Bond Polymer refine detection system DS9800 (Vision Biosystems, Melbourne, Australia).
Even though limited numbers of thyroid LCH with co-existing papillary carcinoma has been reported in the literature [6-10], association with papillary thyroid carcinoma is more often described [11]. However, LCH is very rarely found in lymph nodes draining papillary carcinoma of the thyroid. To the best of our knowledge, only three cases had been reported in the English literature to date [12-14], and this is the first case to be found in Korea [15]. Clinocopathologic features of previously reported cases were described in Table 1. Although one Korean case with multifocal LCH in the thyroid gland has been reported, that case did not showed papillary carcinoma of the thyroid. Moreover, our case is unique because LCH is identified in the thyroid gland and the draining lymph nodes simultaneously. In three previously reported cases, thyroid LCH was not co-existing [12-14].
The etiology of LCH remains unknown. Ambivalence persists as to whether this disorder is primarily neoplastic, immunodysregulatory, or reactive with neoplastic and immunodysregulatory characteristics [16,17]. Many reported cases of LCH of the thyroid have been associated with lymphocytic thyroiditis [3,18]. Coexistence of thyroid LCH and LCH in draining lymph nodes in our case appears to be an incidental finding, even though there remains a possibility that the LCH arose in response to carcinoma and lymphocytic thyroiditis. However, thyroid carcinoma developed years after LCH diagnosis has been made [3]. Thus, thyroid LCH should raise the possibility of other associated disorders.
It has been suggested that LCH cells may alter their migratory properties through the expression of various cytokine receptors and by releasing inflammatory chemokines, causing not only retention of lesional Langerhans cells but also recruitment of eosinophils and T lymphocytes, as well as resultant development of lymphocytic thyroiditis. Lindley et al. [12] reported a very interesting case, which showed that one patient with history of left thyroid lobectomy for papillary carcinoma re-presented with a painful lymph node in the left posterior triangle showing LCH without metastasis two month later. This case suggests that Langerhans cell proliferation may be a reactive phenomenon, probably through the expression of cytokine or chemokine receptors.
In conclusion, LCH associated with papillary thyroid carcinoma and lymph nodes is uncommon. However, it should be borne in mind that LCH in the thyroid gland or lymph nodes can be associated with papillary carcinoma, because LCH may present a diagnostic challenge to the unsuspected pathologist, especially when dealing with aspirates from cervical lymph nodes for metastatic work-up.
Figures and Tables
Fig. 1
Papillary microcarcinoma of the thyroid in the background of lymphocytic thyroiditis (H&E stain, × 40).
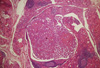
Fig. 2
A. The remainder of the thyroid tissue showed lymphocytic thyroiditis and a separate, incidental, 0.5 cm-sized nodule (left top-arrow) and papillary microcarcinoma is also identified (right bottom-arrowhead) (H&E stain, scanning view, × 12.5). B. Immunohistochemical stain for S100 was strongly positive in the large cells with folded nuclei (× 12.5; inset, × 400), C. while pancytokeratin was C negative (× 12.5).
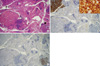
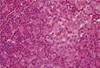
Fig. 4
A. Low-power photograph of a lymph node showing aggregates of pale staining cells (H&E stain, × 100; inset, × 400). B. Sections stained with antibody to S100 protein. There is intense nuclear and cytoplasmic staining of Langerhans cells.

References
1. Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, Bucsky P, Egeler RM, Elinder G, Gadner H, Gresik M, Henter JI, Imashuku S, Janka-Schaub G, Jaffe R, Ladisch S, Nezelof C, Pritchard J. Contemporary classification of histiocytic disorders. The WHO Committee on histiocytic/ reticulum cell proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997. 29:157–166.
2. Fleming MD, Pinkus JL, Fournier MV, Alexander SW, Tam C, Loda M, Sallan SE, Nichols KE, Carpentieri DF, Pinkus GS, Rollins BJ. Coincident expression of the chemokine receptors CCR6 and CCR7 by pathologic Langerhans cells in Langerhans cell histiocytosis. Blood. 2003. 101:2473–2475.
3. Egeler RM, Neglia JP, Puccetti DM, Brennan CA, Nesbit ME. Association of Langerhans cell histiocytosis with malignant neoplasms. Cancer. 1993. 71:865–873.
4. Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis: diagnosis, natural history, management, and outcome. Cancer. 1999. 85:2278–2290.
5. Coode PE, Shaikh MU. Histiocytosis X of the thyroid masquerading as thyroid carcinoma. Hum Pathol. 1988. 19:239–241.
6. Saiz E, Bakotic BW. Isolated Langerhans cell histiocytosis of the thyroid: a report of two cases with nuclear imaging-pathologic correlation. Ann Diagn Pathol. 2000. 4:23–28.
7. Burnett A, Carney D, Mukhopadhyay S, Scalzetti EM, Leino D, Souid AK. Thyroid involvement with Langerhans cell histiocytosis in a 3-year-old male. Pediatr Blood Cancer. 2008. 50:726–727.
8. Foulet-Rogé A, Josselin N, Guyetant S, Gardet JJ, Besancon A, Saint-André JP, Fabiani B. Incidental langerhans cell histiocytosis of thyroid: case report and review of the literature. Endocr Pathol. 2002. 13:227–233.
9. Goldstein N, Layfield LJ. Thyromegaly secondary to simultaneous papillary carcinoma and histiocytosis X. Report of a case and review of the literature. Acta Cytol. 1991. 35:422–426.
10. Thompson LD, Wenig BM, Adair CF, Smith BC, Heffess CS. Langerhans cell histiocytosis of the thyroid: a series of seven cases and a review of the literature. Mod Pathol. 1996. 9:145–149.
11. Vergez S, Rouquette I, Ancey M, Serrano E, Caron P. Langerhans cell histiocytosis of the thyroid is a rare entity, but an association with a papillary thyroid carcinoma is often described. Endocr Pathol. 2010. 21:274–276.
12. Lindley R, Hoile R, Schofield J, Ashton-Key M. Langerhans cell histiocytosis associated with papillary carcinoma of the thyroid. Histopathology. 1998. 32:180.
13. Safali M, McCutcheon JM, Wright DH. Langerhans cell histiocytosis of lymph nodes: draining a papillary carcinoma of the thyroid. Histopathology. 1997. 30:599–603.
14. Schofield JB, Alsanjari NA, Davis J, MacLennan KA. Eosinophilic granuloma of lymph nodes associated with metastatic papillary carcinoma of the thyroid. Histopathology. 1992. 20:181–183.
15. Lee KW, Chung CK, Hwang SC, Yim HH, Park SY, Lee SK, Chung YS, Kim HM, Kim YJ, Hong EK, Chae BN. A case with multifocal Langerhans cell granulomatosis involving the thyroid gland. J Korean Soc Endocrinol. 1998. 13:466–472.
16. Hoover KB, Rosenthal DI, Mankin H. Langerhans cell histiocytosis. Skeletal Radiol. 2007. 36:95–104.
17. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006. 76:363–368.
18. Lieberman PH, Jones CR, Steinman RM, Erlandson RA, Smith J, Gee T, Huvos A, Garin-Chesa P, Filippa DA, Urmacher C, Gangi MD, Sperber M. Langerhans cell (eosinophilic) granulomatosis. A clinicopathologic study encompassing 50 years. Am J Surg Pathol. 1996. 20:519–552.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download