Abstract
Background
17 beta-estradiol is known to play an important role in glucose homeostasis. Lipin-1 is a nuclear protein that is essential in adipocyte differentiation and it is considered to play a role in ectopic fat deposition and the redistribution of fat. The aim of this study was to evaluate the effect of 17 beta-estradiol on the lipin-1 expression in the adipocytes of OLETF rats, which is an animal model of diabetes.
Methods
The OLETF rats were divided into 3 groups, 1) the sham-operation group (SHAM) 2) the castrated group (CAST) and 2) the castrated and estradiol treatment group (EST), and all the rats were at 6 weeks of age. LETO rats were used as a control group (LETO). 0.1 mg of estradiol valerate was injected subcutaneously every 4 weeks in the rats of the EST group. The visceral and subcutaneous tissues were isolated to evaluate the lipin-1 protein expression. The lipin-1 expression was measured in human visceral and subcutaneous preadipocytes.
Results
Less body weight gain was observed in the EST group compared with that of the SHAM group. In addition, improvement in the glucose tolerance was observed in the EST group. The lipin-1 expression in visceral fat was decreased in the SHAM and CAST groups, but it was but recovered in the EST group. The lipin-1 expression in the subcutaneous fat was decreased in the SHAM, CAST, and EST groups.
Figures and Tables
Fig. 1
Body weight changes during experiment. LETO, CAST and EST group shows steadily increase in body weight while SHAM group shows decrease in body weight after 38 weeks. *indicates P value < 0.05 when comparing estrogen treated OLETF rat group with sham operated OLETF rat group. Values are mean ± SE. n = 8 per group.

Fig. 2
Estradiol treated OLETF rats display improved glucose tolerance. *indicates P value < 0.05 when comparing estrogen treated OLETF rat group with sham operated OLETF rat group. Values are mean ± SE. n = 8 per group.

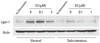
Fig. 3
Protein expression of lipin-1 in visceral and subcutaneous adipose tissues of rats. A. In visceral fat: Lipin-1 expression was decreased by 85% in SHAM and CAST group comparing with LETO group. In EST group, lipin-1 level was restored to 74% of that of LETO group. B. In subcutaneous fat: Lipin-1 expression showed 55% decrease in SHAM group, 72% decrease in CAST group, and 62% decrease in EST group (n = 3-4 for each group).

Fig. 4
Lipin-1 expression in human primary visceral and subcutaneous adipocytes. In visceral adipocyte, lipin-1 expression was increased by 10% with 0.1 uM estrogen and decreased 27% with 1 µM estrogen. In subcutaneous adipocyte, lipin-1 expression was decreased by 26% with 0.1 µM estrogen and decreased 31% with 1 µM estrogen (n = 4).
References
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.
2. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004. 27:1047–1053.
3. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002. 51:7–18.
4. Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001. 44:3–15.
5. Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, Bush TL. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Diabetes Care. 1998. 21:1589–1595.
6. Crespo CJ, Smit E, Snelling A, Sempos CT, Andersen RE. Hormone replacement therapy and its relationship to lipid and glucose metabolism in diabetic and nondiabetic postmenopausal women: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care. 2002. 25:1675–1680.
7. Saglam K, Polat Z, Yilmaz MI, Gulec M, Akinci SB. Effects of postmenopausal hormone replacement therapy on insulin resistance. Endocrine. 2002. 18:211–214.
8. Li C, Samsioe G, Borgfeldt C, Bendahl PO, Wilawan K, Aberg A. Low-dose hormone therapy and carbohydrate metabolism. Fertil Steril. 2003. 79:550–555.
9. Andersson B, Mattsson LA, Hahn L, Mårin P, Lapidus L, Holm G, Bengtsson BA, Björntorp P. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997. 82:638–643.
10. Brussaard HE, Gevers Leuven JA, Frölich M, Kluft C, Krans HM. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia. 1997. 40:843–849.
11. Friday KE, Dong C, Fontenot RU. Conjugated equine estrogen improves glycemic control and blood lipoproteins in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2001. 86:48–52.
12. Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004. 6:180–185.
13. Takiguchi S, Takata Y, Takahashi N, Kataoka K, Hirashima T, Kawano K, Miyasaka K, Funakoshi A, Kono A. A disrupted cholecystokinin A receptor gene induces diabetes in obese rats synergistically with ODB1 gene. Am J Physiol. 1998. 274:E265–E270.
14. Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Prac. 1994. 24:S317–S320.
15. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabet. s. 1992. 41:1422–1428.
16. Shi K, Mizuno A, Sano T, Ishida K, Shima K. Sexual difference in the incidence of diabetes mellitus in Otsuka-Long-Evans-Tokushima-Fatty rats: effects of castration and sex hormone replacement on its incidence. Metabolism. 1994. 43:1214–1220.
17. Phan J, Peterfy M, Reue K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J Biol Chem. 2004. 279:29558–29564.
18. Phan J, Peterfy M, Reue K. Biphasic expression of lipin suggests dual roles in adipocyte development. Drug News Perspect. 2005. 18:5–11.
19. Peterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001. 27:121–124.
20. Xu J, Lee WN, Phan J, Saad MF, Reue K, Kurland IJ. Lipin deficiency impairs diurnal metabolic fuel switching. Diabetes. 2006. 55:3429–3438.
21. Yao-Borengasser A, Rasouli N, Varma V, Miles LM, Phanavanh B, Starks TN, Phan J, Spencer HJ 3rd, McGehee RE Jr, Reue K, Kern PA. Lipin expression is attenuated in adipose tissue of insulin-resistant human subjects and increases with peroxisome proliferator-activated receptor gamma activation. Diabetes. 2006. 55:2811–2818.
22. van Harmelen V, Rydén M, Sjölin E, Hoffstedt J. A role of lipin in human obesity and insulin resistance: relation to adipocyte glucose transport and GLUT4 expression. J Lipid Res. 2007. 48:201–206.
23. Tomiyoshi Y, Sakemi T, Aoki S, Miyazono M. Different effects of castration and estrogen administration on glomerular injury in spontaneously hyperglycemic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Nephron. 2002. 92:860–867.
24. Ocaña A, Gómez-Asensio C, Arranz-Gutiérrez E, Torres C, Señorans FJ, Reglero G. In vitro study of the effect of diesterified alkoxyglycerols with conjugated linoleic acid on adipocyte inflammatory mediators. Lipids Health Dis. 2010. 9:36.
25. Mauvais-Jarvis F, Kulkarni RN, Kahn CR. Knockout models are useful tools to dissect the pathophysiology and genetics of insulin resistance. Clin Endocrinol (Oxf). 2002. 57:1–9.
26. Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, Enzan H, Okada T, Shizuta Y. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest. 2000. 105:1819–1825.
27. Hewitt KN, Pratis K, Jones ME, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004. 145:1842–1848.
28. Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB. Effects of low doses of transdermal 17 beta-estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocrinol Metab. 1992. 74:1396–1400.
29. Paik SG, Michelis MA, Kim YT, Shin S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes. 1982. 31:724–729.
30. Puah JA, Bailey CJ. Insulinotropic effect of ovarian steroid hormones in streptozotocin diabetic female mice. Horm Metab Res. 1985. 17:216–218.
31. Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002. 74:1252–1259.
32. Zhu M, Mizuno A, Kuwajima M, Ogino T, Murakami T, Noma Y, Sano T, Shima K. Ovarian hormone-induced beta-cell hypertrophy contributes to the homeostatic control of beta-cell mass in OLETF female rat, a model of Type II diabetes. Diabetologia. 1998. 41:799–805.
33. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000. 97:12729–12734.
34. Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BH, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000. 97:12735–12740.
35. Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003. 144:1474–1480.
36. O'Sullivan AJ, Ho KK. A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab. 1995. 80:1783–1788.
37. Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007. 13:89–94.
38. Peterfy M, Phan J, Reue K. Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J Biol Chem. 2005. 280:32883–32889.
39. Carman GM, Han GS. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J Biol Chem. 2009. 284:2593–2597.
40. Reue K, Brindley DN. Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J Lipid R. s. 2008. 49:2493–2503.
41. Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC Jr, Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006. 4:199–210.
42. Ohtsuka N, Sakemi T, Tomiyoshi Y, Morito F. Attenuating effect of castration or estrogen administration on glomerular injury in adriamycin-induced nephropathy. Nephrology. 1996. 2:45–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download