Abstract
Objective
The aim of the study was to investigate whether lithium chloride and medroxyprogesterone acetate can potentiate the cytotoxicity of imatinib mesylate in human endometrial cancer in vitro and the effect of midkine in these therapies.
Methods
Imatinib mesylate (50 µM), lithium chloride (100 µM), medroxyprogesterone acetate (200 µM) and their combination were applied to monolayer and three dimensional cultures of human Ishikawa endometrial cancer for 72 hours. The cell proliferation index, apoptotic index, caspase-3 and midkine levels, cell cycle distributions in monolayer cultures and cell ultrastructure in spheroid cultures were evaluated. Results were statistically analyzed using the Student's t-test.
Results
All drug applications inhibited cell proliferation (p<0.05), however the combination were the effective groups for 72 hours (p<0.05). Interestingly, although the loss of efficiency was seen higly seen every 24 hours at single applications, the inhibition rates of the combination groups were almost same for 72 hours. In concordance with these results, the apoptotic index, caspase-3 levels (p<0.05), cell morphology and ultrastructure damages were much higher in the combination groups. Imatinib mesylate induced S-phase arrest, however other groups induced G0+G1-phase arrest at 24 hours and all groups induced G0+G1 arrest at 72 hours (p<0.05). Imatinib mesylate and imatinib mesylate with medroxyprogesterone acetate induced highest decrease in midkine levels, respectively (p<0.05).
Endometrial carcinoma is the most common type of uterine cancer and is a frequently seen gynecologic malignancy with breast cancer in developed countries. Patients with advanced or recurrent disease have poor prognosis sucha that it can be very hard to cure by surgery, conventional chemotherapy, radiation or a combination of these modalities. As some estimate that the percent of women dying from endometrial carcinoma has increased by 227% over the past decade, there is a great need to develop novel targeted agents that can be used alone or in combination with current treatment strategies [1].
Endometrial cancers are divided into two types (type I and type II) based on their biological, molecular and clinical parameters [2]. Eighty percent of cases consist of type I or those tumors of endometrioid histology. Previous studies showed that they arise from persistent unopposed estrogen stimulation and in addition, they are generally estrogen receptor and progesterone receptor positive. The underlying mechanisms can be listed as genetic alterations associated with these tumors include PTEN deletions or mutations (36-83%), microsatellite instability (20-40%), mutations of K-ras (15-30%) and gain of function mutations in β-catenin (25-40%) [3,4].
Imatinib mesylate (IM) also called Gleevec or STI571 is a well known tyrosine kinase inhibitor that has been used as a "magic bullet" for the treatment of leukemia and gastrointestinal tumours. IM inhibits the activity of a number of receptor tyrosine kinases as Abelson cytoplasmic tyrosine kinase (ABL), c-Kit, the platelet-derived growth factor receptor, and epidermal growth factor receptor (EGFR) [5]. Its success led the investigators to search its antineoplastic effects on other cancer types including endometrial cancer [6-9]. Previous reports that studied IM demonstrated that endometrial cancers which expressed IM targets respond to IM treatment and in these reports they also mentioned that IM should be used in combination in order to make the treatment more efficient [6,7].
Lithium chloride (LiCl) has been used clinically since the 19th century to treat psychotic diseases as bipolar disorders, and its safety profile is well documented. Previous studies showed that LiCl also possess antineoplastic effects in a variety of cancers including colorectal cancer [10], gastric cancer [11], and neuroblastoma [12]. It was shown that anti-neoplastic action of LiCl primarily depends on the inhibition of glycogen synthase kinase 3-beta [13].
Medroxyprogesterone acetate (MPA) is a synthetic, orally active derivative of the natural steroid hormone progesterone, widely used in contraception of women/men and in oncology, both in the endocrine treatment of hormone-related cancers and as supportive therapy in the cachexia syndrome [14,15]. MPA is the only approved drugs in Europe for the clinical treatment of cancer-related anorexia/cachexia syndrome at the moment [16]. The anti-contraceptive and anti-neoplastic effects of MPA are the results of the anti-estrogenic action of MPA [17]. In the present study we focused on its antineoplastic effect. Wang et al. [18] proposed that Wnt/β-catenin signaling is activated by estrogen and inhibited by progesterone during the menstrual cycle. When this balanced is disrupted, the enhanced or unopposed estrogen signalling may lead to constitutive activation of Wnt/β-catenin signaling and this will trigger endometrial hyperplasia, which may develop further into endometrial cancer. In addition, the direct effect of enhanced or unopposed estrogen to nucleus through its receptor is the another reason for endometrial transformation to cancer.
Midkine (MDK), a newly pronounced old molecule, is a heparin-binding growth factor playing a central role in carcinogenesis as an antiapoptotic and angiogenic factor. High expression of MDK in various human tumors and the success gained after inhibition of MDK make it a promising target for specific therapies [19]. It has been proven to be involved in tumorigenesis of neuroblastoma, astrocytoma and malignant peripheral nerve sheath tumors and an elevated MDK expression has been detected in tumors such as the colon, lung, pancreas, stomach, esophageal tumors, hepatocellular carcinoma, and endometrial carcinoma [19]. It was shown that treatment of normal endometrial cells by 17-beta oestradiol increased MDK levels [20].
In the present study we investigated whether LiCl and MPA can potentiate the cytotoxicity of IM in treatment of endometrial cancer and the effect of MDK in these therapies. We targeted to treat both multiple diseases and increase life quality at same time. This means LiCl may increase IM cytotoxicity, treat another type of cancer (primary and secondary) and psychiatric disorder as bipolar disorder at the same time, on the another side different from LiCl, MPA can also treat cancer-related cachexia/anorexia and prevent pregnancy.
Ishikawa cells (a generous gift from Uludag University, Bursa, Turkey) were routinely maintained in phenol-red-free RPMI 1640 (Sigma medium containing 10% foetal calf serum [FCS], Sigma, St. Louis, MO, USA) and 2 mM glutamine (Gmax, Sigma) and incubated at 37℃ with 5% CO2 in 75 cm2 flasks (TPP). For all dosing experiments, the medium was replaced with RPMI 1640 containing 10% charcoal-stripped FCS (CSS, Hyclone) and 2 mM glutamine (Gmax) for 72 hours prior to treatment. All experiments were performed in triplicate on Ishikawa cells between passage number 3 and 15 and repeated three times.
An in vitro multicellular Ishikawa spheroid model was established using a liquidoverlay technique. Briefly, semi-confluent monolayer cell cultures were trypsinized and single cells with 100% vitality were cultured over 3% Noble agar-coated (Difco, Franklin Lakes, NJ, USA) six-well culture plates containing 5 mL RPMI-1640 medium at a concentration of 1×106 cells/well.
IM (IC50, 50 µM), LiCl (IC50, 100 µM), and MPA (IC50, 200 µM) and their combination were applied to monolayer and spheroid cultures of estrogen- and progesterone-positive human Ishikawa endometrium cells for 72 hours. The cell proliferation index, apoptotic index and cell cycle distributions by flow cytometry, morphology by scanning electron microscopy in monolayer cultures and cell ultrastructure by transmission electron microscopy (TEM) in three dimensional cultures were evaluated for 72 hours. Results were statistically analyzed using the Student's t-test.
The total cell number was counted by using an automated cell counter (NucleoCounter, ChemoMetec A/S, Allerod, Denmark). The starter kit which is compatible to cell counter and includes lysis buffer, stabilization buffer, nucleocasettes and software was used. Cells were harvested every 24 hours for 72 hours. Cells were pre-treated with lysis and stabilization buffers to dissolve cell aggregates and lyse cell membranes. Pre-treated cells were loaded to nucleocasettes which were coated with propidium iodid (PI) dye and their nuclei was stained with PI. Nucleocasettes were placed in device for 30-35 seconds to measure the PI fluorescence and then cell counts were analyzed with the software and recorded.
The apoptotic index was evaluated by using flow cytometric Annexin-V-fluorescein isothiocyanate/propidium iodide (Annexin-V-FITC/PI) staining. Following the instruction manual of the kit (BD Pharmingen, San Diego, CA, USA), briefly, cells were washed twice with PBS and resuspended by binding buffer containing 0.01 M HEPES, 0.14 mM NaCl, and 2.5 mM CaCl2. A cell suspension (1×105 cells in 100 µL) in binding buffer was incubated with 5 µL of FITC-labeled Annexin V (BD Pharmingen) dye and PI for 15 minutes in the dark at room temperature. After incubation, the PI fluorescence and Annexin V were measured simultaneously in a BD FACS Calibur and analyzed with the instrument's operating software (CellQuest: BD Pharmingen). Data acquisition and analysis were undertaken with CellQuest and WinMDI programs.
Caspase-3 levels in triplicate were analyzed using fluorimetric kits (Sigma Aldrich, St. Louis, MO, USA). The caspase-3 fluorimetric assay is based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) by caspase 3, resulting in the release of the fluorescent 7-amino-4-methylcoumarin (AMC) moiety. 1×104 cells seeded in each well of 96 well plates washed twice in PBS and incubated in CHAPS lysis buffer at 4℃ for 20 minutes. We transfered 5 µL of cell lysate into the wells of other 96 well plates, then incubated with 5 µL of 2 mM Ac-DEVD-pNA peptide substrate and 200 µL of assay buffer (HEPES 20 mM, pH 7.4, CHAPS 0.1%, DTT 5 mM, EDTA 2 mM) at 37℃ for 1 hour in an incubator. The concentration of AMC released was quantified by reading in a fluorometer with a 360 nm excitation filter and 460 nm emission filter for optimal sensitivity.
The effects of drugs on the cell cycle were examined using a DNA analysis kit (BD Pharmingen) according to the manufacturer's instructions. Briefly, were induced at a cell density of 51×105 cells/ml in the presence of each drug applied separately and in combination for different time intervals (24 and 72 hours). Ishikawa cells were harvested, centrifuged, washed and resuspended in buffer (dimethylsulfoxide in sucrose-sodium citrate) for 5 minutes at room temperature, respectively. A mixture of trypsin in spermine tetrahydrochloride detergent buffer was added and samples were incubated for 20 minutes at room temperature. After the addition of RNaseA and trypsin inhibitor in spermine buffer, cells were incubated with propidium iodide, in dark, for 20 minutes at 4℃. Finally, flow cytometric analysis was performed immediately using a Facscan flow cytometer (FACS Diva, Beckman-Dickinson, San Jose, CA, USA) and fluorescence intensity data were acquired using the instrument's operating software (CellQuest). The percentages of the analyzed cell population in G0/G1-, S- or G2/M-phases were determined by the Mod Fit cell-cycle analysis program.
Harvested spheroids were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer and post-fixed in 1% osmium tetraoxide in 0.1 M sodium cacodylate buffer for 1 hour at 4℃. Cells were incubatedin 1% uranyl acetate for 1 hour at 4℃, dehydrated in a graded acetone series and embedded in Epon 812. Samples were cut using a rotating blade microtome (Leica, Heerbrugg, Switzerland) and 70 nm-thick sections were mounted on copper grids. Sections were subsequently stained with 5% uranyl acetate and counterstained with Reynold's lead citrate. Sections were examined with a Jeol-Jem 1011 transmission electron microscope. Photographs were taken at several magnifications.
Cell culture supernatants were analyzed for midkine levels in triplicate using ELISA kits (PeproTech, Rocky Hill, NJ, USA). The lower detection limit of the assay was 150 pg/mL for midkine. Midkine levels were measured by an ELISA system in which polyclonal antihuman midkine was used as capture antibody (Peprotech). Detection was bybiotinylated polyclonal antihuman midkine antibody (PeproTech) followed by streptavidin HRP (Sigma) and a TMB enzyme substrate system (Sigma). The reaction was stopped by 1 M H2SO4 and readings were made at 450 nm by a spectrometer (M2, Molecular devices, Sunnyvale, CA, USA).
SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) and Excel 2007 were used for the statistical analysis. All results were statistically analyzed using the Student's t-test. Data were represented as mean±SE. A p<0.05 was considered significant. Synergy was determined as described previously [21]. Briefly, synergism was determined using the following formula: Combination index (CI): D1/(DX)1+D2/(DX)2 where D1 is tested concentration of IM used in combination with LiCl or MPA, D2 is the tested concentration of LiCl or MPA used in combination with IM, (DX)1 is the concentration of a singly applied IM and (DX)2 is the concentration of a singly applied LiCl or MPA. A CI value of 1 indicates an additive effect, a CI value <1 indicates a synergistic effect and a CI value >1 indicates an antagonist effect.
As shown in Fig. 1, all drug treatments decreased cell numbers (p<0.05). Singly applied drugs lost their efficiency in a time dependent manner, however the combination groups did not lose their efficiency and led to a high decrease in cell number for 72 hours (p<0.05). MPA and the combination of IM and MPA seemed to be the most efficient drug applications for 72 hours (p<0.05).
Fig. 2 showed that all drug treatments increased the apoptotic index (p<0.05). Apoptotic index of singly applied drugs decreased in a time dependent manner, but the apoptotic index induced by MPA was the highest (p<0.05). The combination groups induced higher apoptotic index than singly applied drugs and IM with MPA induced the highest apoptotic index (p<0.05).
Caspase-3 levels in Fig. 3 showed that all drug treatments increased apoptosis (p<0.05). The increase in caspase-3 levels were determined both at single and combined drug applications (p<0.05). The highest increase were determined at the combination groups (p<0.05). MPA and IM with MPA induced the highest caspase-3 levels (p<0.05).
LiCl, MPA and the combination groups led to G0+G1 arrest at 24 hour except for IM. IM induced S-phase arrest at this time interval. However, all drug applications induced G0+G1 arrest at 48 hours and 72 hours (Fig. 4).
Fig. 5 showed that all drug applications induced a decrease in MDK levels for 72 hours (p<0.05). Highest decrease was determined at IM combined with MPA and IM combined with LiCl, respectively for 72 hours (p<0.05). Among single applied drugs, IM induced highest decrease and the latter were MPA and then LiCl (p<0.05).
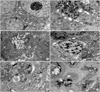
According to the TEM results in Fig. 6, the cells of the control group presented healthy morphology including intact cell membrane with microvilli, cell junctions, intact nuclear membranes with the proportional spread euchromatin, the proportional distribution of nucleus and cytoplasm, mitochondria in a normal size with intact outer and inner cell membranes, cytoplasmic reticulums and ribosomes in a normal size. The intranuclear channel formation and lipid vacuoles were determined in some cells of spheroids (Fig. 6A). After IM was applied, disrupted cell mebranes, apparent cell apoptosis was observed, i.e. chromatins aggregated around nuclear membrane, intact nuclear membrane, vacuoles with unknown content, cell remnants were determined between intercellular area. Gaps were formed inside the spheroids due the loss of cell interractions (Fig. 6B). LiCl applied spheroids showed that intact cell and nuclear membranes, the loss of cell to cell interactions, lytic cytoplasm with many vacuoles with unknown content and autophagic vacuoles, many lipid vacuoles (Fig. 6C). The spheroids of the combination group IM with LiCl showed high apoptotic appearence, gaps inside spheroids due to the loss of cell interactions, severe mitochondri damage, i.e, swollen mitochondria with disrupted inner membranes, vacuoles. Some cells lost their cell membrane, consequently cell remnants can be determined in the intercellular area (Fig. 6D). MPA induced the loss of cell interactions, apoptotic nucleus, many auotophagic and lipid vacuoles, swollen mitochondria with disrupted and/or lost inner membranes (Fig. 6E). No intact spheroid structure was determined in the combination of IM with MPA. High incidence of cell remnants were seen in the intercellular area. The morphological changes which were observed in rarely seen cells were listed as the loss of nucleus membranes, high apoptotic appearence, severe mitochondria damage with disrupted and/or lost outer and inner membranes, and huge autophagic vacuoles (Fig. 6F).
The Ishikawa human endometrial carcinoma cell line has both estrogen and progesteron receptors like type I endometrium carcinoma [2]. We planned to prevent/inhibit estrogen effects by using a hormone MPA and a chemical LiCl. Both two different treatments had common targets to inhibit estrogen effect. According to our results, we detected the highest efficiency in the single applied MPA and its combination with IM. Although single LiCl took third place in efficient inhibition among singly applied drugs and lost its effect much more than others as time advanced, its combination with IM was very effective and did not lose its effect in a time dependent manner.
IM was the second effective drug after MPA among the single applied drugs in the present study. As documented before, the mitogenic action of estrogen in the endometrial cancer through growth factors and their receptors (EGFR, IGF-IR, c-MET) comprise the activation of two key signaling cascades as the PI3K/AKT and the RAS/RAF/MAPK pathways. In addition, it was proposed that both estrogen receptor alpha (ERα) and AKT play a double role as both downstream target and activate each other. AKT-mediated phosphorylation of ERα results in the transcriptional activation of ERα, independent of ligand binding [2]. IM may terminate one of the estrogen mediated mitogenic signalling through the inhibition of receptor tyrosine kinases in this study.
Flow cytometric apoptotic index, caspase-3 levels and ultrastructure analysis showed that the reason for the cell proliferation inhibition and the disruption of spheroid structure was apoptotic cell death. However, ultrastructure analysis of MPA and its combination with IM gave additional information that the autophagic cell death may take part in their mechanism of action. In our previous study with MPA, the FM3A murine breast tumor cell line was treated with epirubicin alone and with MPA or tamoxifen, and we determined that all drugs induced autophagy, but when tamoxifen combined with MPA autophagy was increased [22]. Different from single MPA, autophagic vacuoles which were observed in the combintion group were huge. In contrast to our previous studies in neurologic tumours [23], no autophagic vacuoles were determined in the IM group. Recent reports mentioned that autophagy is a two-edged sword that can lead to cell survival or cell death (autophagic cell death). Orrenius et al. [24] suggested that there is a cross-talk between cell death modalities, and this means different signals can cause a shift from autophagy to apoptosis or apoptosis to autophagy, or a mixture of these two cell-death modes. In the light of the increased efficiency of the combination group, we suggest that autophagic vacuoles may belong to the autophagic cell death, which can exist at the same time with apoptotic cell death or can be pre-step for the apoptotic cell death.
Nishio et al. [14] treated two cases of multidrug-resistant recurrent endometrial cancer with MPA successfully. Although they accomplished complete response after surgical operation and post operative chemotherapy with MPA for endometrial cancer, they found lung and small intestine metastasis. Eventually, they performed surgical and postoperative therapy again. The combination of MPA with IM can treat both the primary tumor as endometrial cancer and metastic tumours as gastrointestinal stromal tumors (GISTs) etc. MPA can be used when the hormone receptor status are positive. In the present study, we also used LiCl without taking into consideration the estrogen and its receptor status to determine the treatment fate in MPA resistant tumours. The efficiency of LiCl with IM was also very effective in a time dependent manner similar to MPA with IM. It can be proposed that IM with LiCl can also be used effectively in MPA resistant tumours and their metastasis.
Rawnaq et al. [25] in serum of patients with GISTs and Erguven et al. [unpublished observation] in human glioblastoma cells in vitro showed that IM induced decrease in MDK levels. Concomitant with this study, in the present study we determined that IM decreased MDK levels and it was the most efficient group. Zhang et al. [20] examined the expression of the mRNA coding for seven polypeptide angiogenic factors in normal endometrial epithelial, stromal and three endometrial carcinoma lines. The endometrial epithelial and stromal cells express mRNA for the polypeptide angiogenic factors, basic fibroblast growth factor, vascular endothelial cell growth factor, transforming growth factor-beta 1 and pleiotrophin, as well as the cytokine midkine. They stimulated growth of normal endometrial epithelial cells by 17-beta-oestradiol and epidermal growth factor. They determined that expression of the mRNA of both vascular endothelial growth factor and MDK in normal endometrial epithelial cells showed a 2-fold increase after treatment with a physiological dose of 17-beta-oestradiol (10-10 M), while, in contrast, the mRNA of transforming growth factor-beta 1 decreased 4-fold after treatment with 17-beta-oestradiol (10-10 M) and was abolished by exposure to progesterone (5×10-9 M) [21]. In our study, concomitant with Zhang et al. [20], MPA decreased MDK levels efficiently after IM, and IM with MPA induced the highest decrease in MDK levels among all groups. As in our recent study in human neuroblastoma cell line named SH-SYSY [12], we determined that LiCl decreased MDK levels in Ishikawa human endometrium cancer cells and second highest decrease in MDK levels were determined with the combination of IM and LiCl. According to our results, it may be concluded that high MDK level decrease in combination groups were determined because of IMs' highest activity.
We concluded that LiCl and MPA potentiated the cyctotoxicity of IM, and the inhibition of estrogens activity through growth factors including MDK, wnt/catenin pathway may be involved in termination of endometrial cancer defense. This multi-targeted therapies may provide treatment for resistant, metastatic and recurrent endometrial cancers with anorexia/cachexia, physcotic disorders and contraception problems. Eventually, the treatment of cancer with/without complications with regained/increased life quality can be accomplished at the end of this protocol. Further investigations with different human endometrial carcinoma cell lines in vitro and in vivo are needed to start clinical trials.
Figures and Tables
Fig. 1
The effects of single and combined drugs on cell proliferation. Columns, average of six wells. Data are representative of separate three experiments. C, control; IM, imatinib mesylate; LiCl, lithium chloride; IM+LiCl, the combination of IM and LiCl; MPA, medroxyprogesterone acetate; IM+MPA, the combination of IM and MPA.

Fig. 2
The alterations in apoptotic index induced by different drug applications. Columns, average of six wells. Data are representative of separate three experiments. C, control; IM, imatinib mesylate; LiCl, lithium chloride; IM+LiCl, the combination of IM and LiCl; MPA, medroxyprogesterone acetate; IM+MPA, the combination of IM and MPA.

Fig. 3
Caspase-3 levels effected by single and combined drugs. Columns, average of six wells. Data are representative of separate three experiments. C, control; IM, imatinib mesylate; LiCl, lithium chloride; IM+LiCl, the combination of IM and LiCl; MPA, medroxyprogesterone acetate; IM+MPA, the combination of IM and MPA.

Fig. 4
The alterations of cell cycle distributions by different drug applications. Columns, average of six wells. Data are representative of separate three experiments. C, control; IM, imatinib mesylate; LiCl, lithium chloride; IM+LiCl, the combination of IM and LiCl; MPA, medroxyprogesterone acetate; IM+MPA, the combination of IM and MPA.

Fig. 5
The effect of different drug applications on MDK levels. Columns, average of six wells. Data are representative of separate three experiments. C, control; IM, imatinib mesylate; LiCl, lithium chloride; IM+LiCl, the combination of IM and LiCl; MPA, medroxyprogesterone acetate; IM+MPA, the combination of IM and MPA.

Fig. 6
The alterations in cell ultrastructure by different drug applications. (A) Control (×7,500), (B) Imatinib mesylate (IM; ×10 k), (C) Lithium chloride (LiCl; ×6,000 and ×6,000), (D) IM+LiCl (×10 k and ×7,500), (E) Medroxyprogesterone acetate (MPA; ×7,500), (F) IM+MPA (×10 k and ×7,500). n, nucleus; nu, nucleolus; lp, lipid vacuole; mi, mitochondria; mv, microvillus; lt, lytic cytoplasm; j, junctions; v, vacuole; ch, chromatin; cr, cell remnants; *, apoptotic cell; av, autophagic vacuole; g, gaps.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.
2. Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol. 2007. 2:57–85.
3. Abal M, Planaguma J, Gil-Moreno A, Monge M, Gonzalez M, Baro T, et al. Molecular pathology of endometrial carcinoma: transcriptional signature in endometrioid tumors. Histol Histopathol. 2006. 21:197–204.
4. Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004. 444:213–223.
5. Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2010. 184:3–20.
6. Salvatierra A, Tarrats A, Gomez C, Sastre JM, Balana C. A case of c-kit positive high-grade stromal endometrial sarcoma responding to Imatinib Mesylate. Gynecol Oncol. 2006. 101:545–547.
7. Mitsuhashi T, Nakayama M, Sakurai S, Fujimura M, Shimizu Y, Ban S, et al. KIT-negative undifferentiated endometrial sarcoma with the amplified epidermal growth factor receptor gene showing a temporary response to imatinib mesylate. Ann Diagn Pathol. 2007. 11:49–54.
8. Ha HT, Lee JS, Urba S, Koenig RJ, Sisson J, Giordano T, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010. 20:975–980.
9. Ustach CV, Huang W, Conley-LaComb MK, Lin CY, Che M, Abrams J, et al. A novel signaling axis of matriptase/PDGF-D/β-PDGFR in human prostate cancer. Cancer Res. 2010. 70:9631–9640.
10. Vidal F, de Araujo WM, Cruz AL, Tanaka MN, Viola JP, Morgado-Diaz JA. Lithium reduces tumorigenic potential in response to EGF signaling in human colorectal cancer cells. Int J Oncol. 2011. 38:1365–1373.
11. Cho YJ, Kim JH, Yoon J, Cho SJ, Ko YS, Park JW, et al. Constitutive activation of glycogen synthase kinase-3beta correlates with better prognosis and cyclin-dependent kinase inhibitors in human gastric cancer. BMC Gastroenterol. 2010. 10:91.
12. Bilir A, Erguven M, Yazihan N, Aktas E, Oktem G, Sabanci A. Enhancement of vinorelbine-induced cytotoxicity and apoptosis by clomipramine and lithium chloride in human neuroblastoma cancer cell line SH-SY5Y. J Neurooncol. 2010. 100:385–395.
13. Zhu H, Han B, Pan X, Qi H, Xu L. Thiazolidenediones induce tumour-cell apoptosis through the Akt-GSK3β pathway. J Clin Pharm Ther. 2011. 03. 16. [Epub]. http://dx.doi.org/10.1111/j.1365-2710.2011.01251.x.
14. Nishio S, Koyanagi T, Miyabe K, Kuromatsu H. Two cases of multidrug-resistant recurrent endometrial cancer successfully treated with medroxyprogesterone acetate (MPA). Gan To Kagaku Ryoho. 2010. 37:735–738.
15. Cade TJ, Quinn MA, Rome RM, Neesham D. Progestogen treatment options for early endometrial cancer. BJOG. 2010. 117:879–884.
16. Madeddu C, Maccio A, Panzone F, Tanca FM, Mantovani G. Medroxyprogesterone acetate in the management of cancer cachexia. Expert Opin Pharmacother. 2009. 10:1359–1366.
17. Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011. 22:145–152.
18. Wang Y, van der Zee M, Fodde R, Blok LJ. Wnt/B-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget. 2010. 1:674–684.
19. Muramatsu T. Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2010. 86:410–425.
20. Zhang L, Rees MC, Bicknell R. The isolation and long-term culture of normal human endometrial epithelium and stroma: expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J Cell Sci. 1995. 108:323–331.
21. Miller MC 3rd, Johnson KR, Willingham MC, Fan W. Apoptotic cell death induced by baccatin III, a precursor of paclitaxel, may occur without G(2)/M arrest. Cancer Chemother Pharmacol. 1999. 44:444–452.
22. Bilir A, Altinoz MA, Erkan M, Ozmen V, Aydiner A. Autophagy and nuclear changes in FM3A breast tumor cells after epirubicin, medroxyprogesterone and tamoxifen treatment in vitro. Pathobiology. 2001. 69:120–126.
23. Erguven M, Yazihan N, Aktas E, Sabanci A, Li CJ, Oktem G, et al. Carvedilol in glioma treatment alone and with imatinib in vitro. Int J Oncol. 2010. 36:857–866.
24. Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011. 119:3–19.
25. Rawnaq T, Kunkel M, Bachmann K, Simon R, Zander H, Brandl S, et al. Serum midkine correlates with tumor progression and imatinib response in gastrointestinal stromal tumors. Ann Surg Oncol. 2011. 18:559–565.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download