Abstract
Purpose
Materials and Methods
Results
Notes
AUTHOR CONTRIBUTIONS:
Conceptualization: You Jin Chun, Hei-Cheul Jeung, Tae Joo Jeon.
Data curation: You Jin Chun, Hei-Cheul Jeung, Tae Joo Jeon.
Formal analysis: You Jin Chun.
Funding acquisition: Hei-Cheul Jeung, Tae Joo Jeon.
Investigation: You Jin Chun, Hei-Cheul Jeung, Tae Joo Jeon.
Methodology: You Jin Chun, Tae Joo Jeon.
Project administration: You Jin Chun.
Resources: You Jin Chun, Hyung Soon Park, Ji Soo Park, Sun Young Rha, Hye Jin Choi.
Software: Jae-Hoon Lee, Tae Joo Jeon.
Supervision: Hei-Cheul Jeung, Tae Joo Jeon.
Validation: Tae Joo Jeon.
Visualization: You Jin Chun.
Writing—original draft: You Jin Chun.
Writing—review & editing: You Jin Chun, Hei-Cheul Jeung, Tae Joo Jeon.
References
Fig. 1
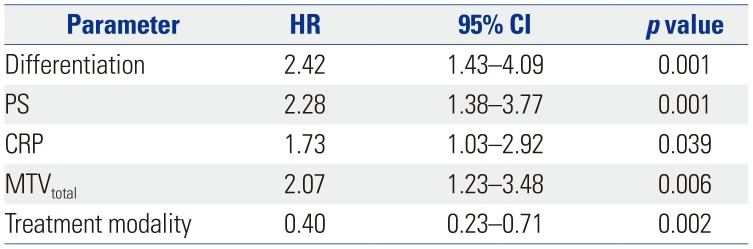
Kaplan-Meier curves for overall survival based on significant prognostic factors, including (A) pathologic differentiation [HR=2.42 (well differentiated and moderately differentiated vs. poorly differentiated); p=0.001], (B) performance status [HR=2.28 (ECOG 0, 1 vs. 2, 3); p=0.001], (C) CRP [HR=1.73 (≤6 mg/dL vs. >6 mg/dL); p=0.039], and (D) MTVtotal [HR=2.07 (≤350.77 cm3 vs. >350.77 cm3); p=0.006] in gallbladder carcinoma. ECOG, Eastern Cooperative Oncology Group; CRP, C-reactive protein; MTVtotal, the sum of the MTVs of both the locally advanced and metastatic lesions; HR, hazard ratio.
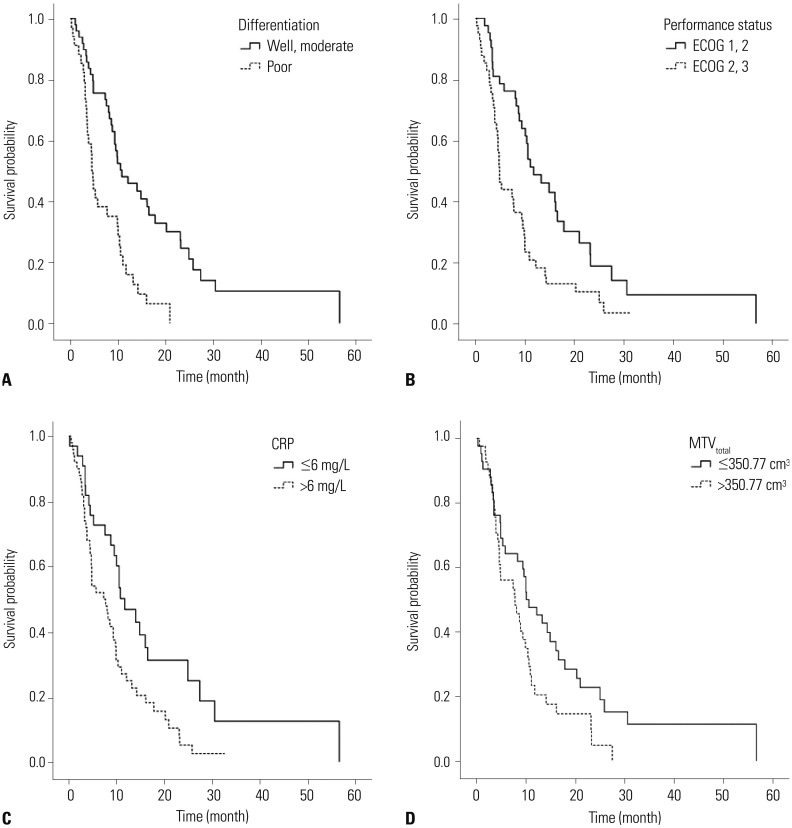
Table 1
Baseline Patient Characteristics with Positron Emission Tomography Parameters of Primary and Metastatic Lesions
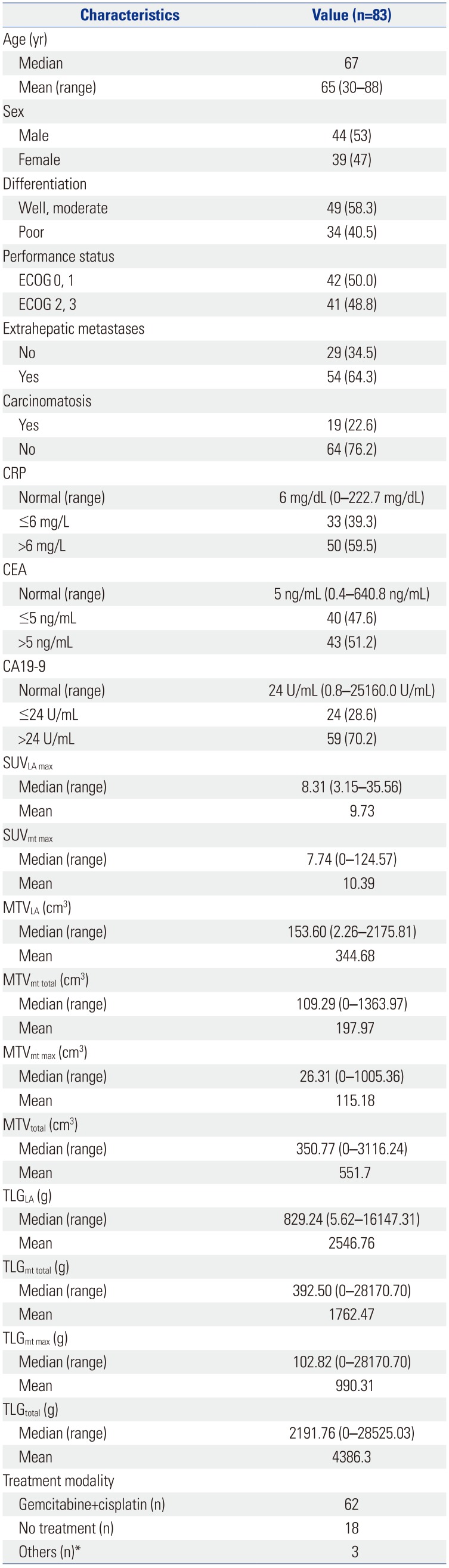
ECOG, Eastern Cooperative Oncology Group; CRP, C-reactive protein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; SUV, standardized uptake value; SUVLA max, the highest SUV of the locally advanced lesion; SUVmt max, the highest SUV among the distant metastatic lesions; MTV, metabolic tumor volume; MTVLA, the MTV of the locally advanced lesion; MTVmt total, the sum of the MTVs of all metastatic lesions; MTVmt max, the highest MTV among the metastatic lesions; MTVtotal, the sum of the MTVs of both the locally advanced and metastatic lesions; TLG, total lesion glycolysis; TLGLA, the TLG of the locally advanced lesion; TLGmt total, the sum of the TLGs of all metastatic lesions; TLGmt max, the highest TLG among the TLGmt; TLGtotal, the sum of the TLGs of both locally advanced and metastatic lesions.
Values are presented as number (percentage) unless otherwise noticed.
*Others, one patient with concurrent chemotherapy (CCRT) using cisplatin, one patient with CCRT using fluorouracil, and one patient with radiotherapy alone.
Table 2
Univariate Analysis of Prognostic Factors for Survival Outcomes
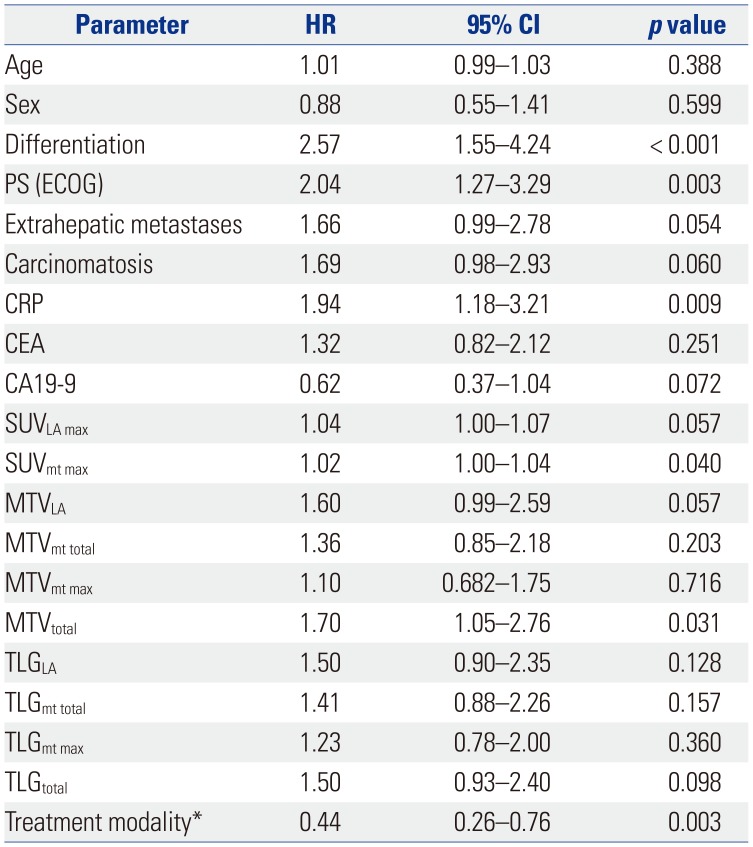
HR, hazard ratio; CI, confidence interval; PS, performance status; ECOG, Eastern Cooperative Oncology Group; CRP, C-reactive protein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; SUV, standardized uptake value; SUVpri max, the highest SUV of the primary lesion; SUVmt max, the highest SUV among the metastatic lesions; MTV, metabolic tumor volume; MTVpri, the MTV of the primary lesion; MTVmt total, the sum of the MTVs of all metastatic lesions; MTVmt max, the highest MTV among the metastatic lesions; MTVtotal, the sum of the MTVs of both the primary and metastatic lesions; TLG, total lesion glycolysis; TLGpri, the TLG of the primary lesion; TLGmt total, the sum of the TLGs of all metastatic lesions; TLGmt max, the highest TLG among the TLGmt; TLGtotal, the sum of the TLGs of both primary and metastatic lesions.
*Sixty-two patients received gemcitabine plus cisplatin chemotherapy, 18 patients received no treatment, and 3 patients received concurrent chemotherapy or radiotherapy alone.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download