Abstract
Purpose
Materials and Methods
Results
References
Fig. 1
Flow diagram of the study population. A total of 58 patients were enrolled in this study and divided into two groups. PFT, pulmonary function test; FEV1, forced expiratory volume in 1 second.
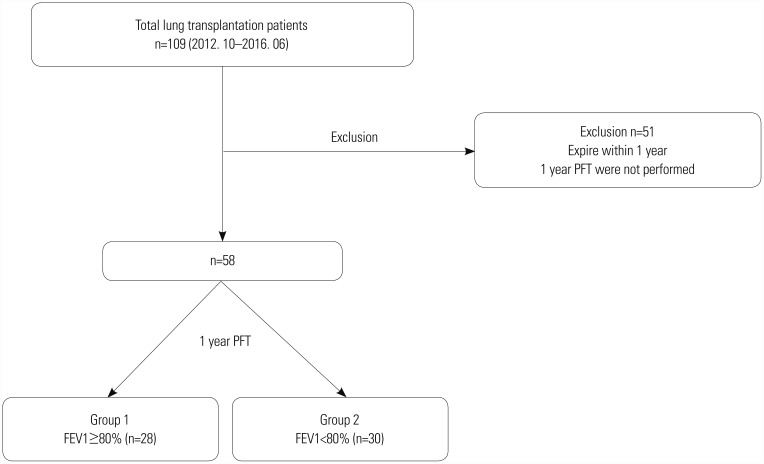
Fig. 2
Recovery of pulmonary function for 1-year after transplantation. After transplantation, pulmonary function tests were performed at 1, 3, 6, 9, and 12 months after transplantation. Postoperative FVC (A and B), FEV1 (C and D), and FEF 25–75% (E and F) recovered significantly better in the FEV1 ≥80% group.
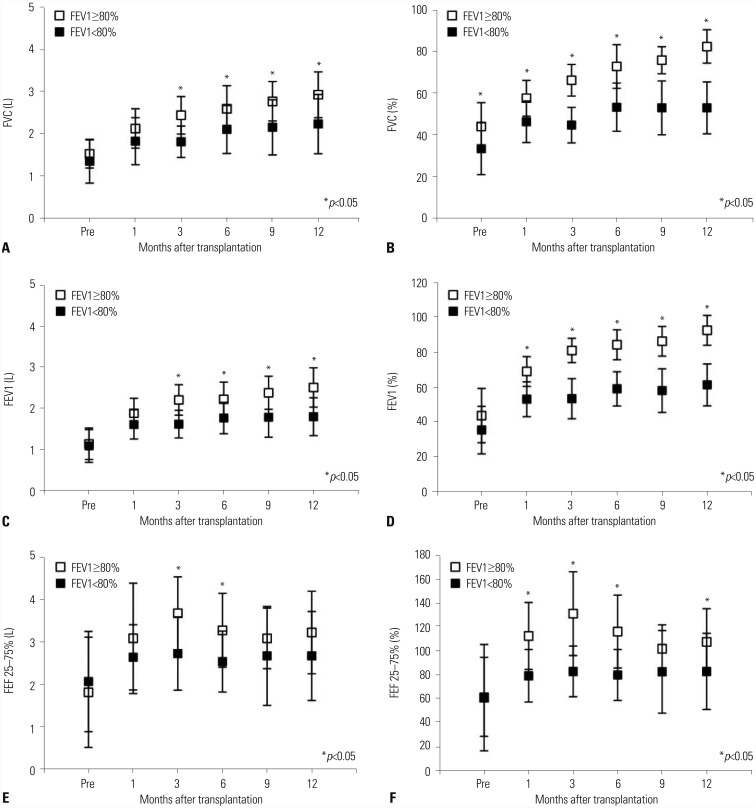
Table 1
Baseline Characteristics of the Recipients
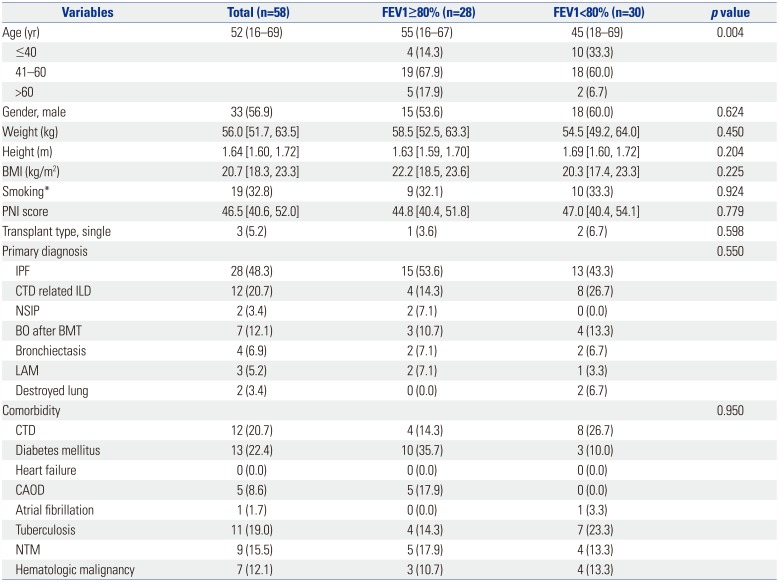
FEV1, forced expiratory volume in 1 second; BMI, body mass index; PNI, prognostic nutritional index; IQR, interquartile range; IPF, idiopathic pulmonary fibrosis; CTD related ILD, connective tissue disease related interstitial lung disease; NSIP, nonspecific interstitial pneumonia; BO, bronchiolitis obliterans; BMT, bone marrow transplantation; LAM, lymphangioleiomyomatosis; CTD, connective tissue disease; CAOD, coronary artery occlusive disease; NTM, nontuberculosis mycobacterium.
Data are presented as numbers (percentage), median (range), or median [interquartile range].
*≥20 pack year (PYR).
Table 2
Univariate Analysis of Pulmonary Function Tests
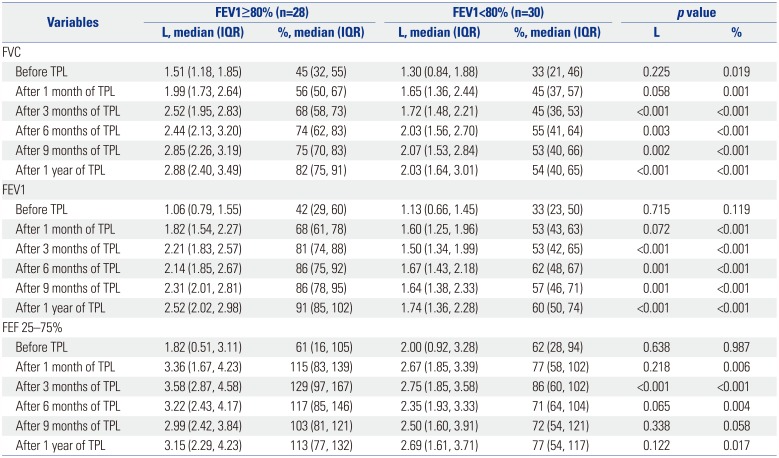
Table 3
Univariate Analysis of Perioperative Variables
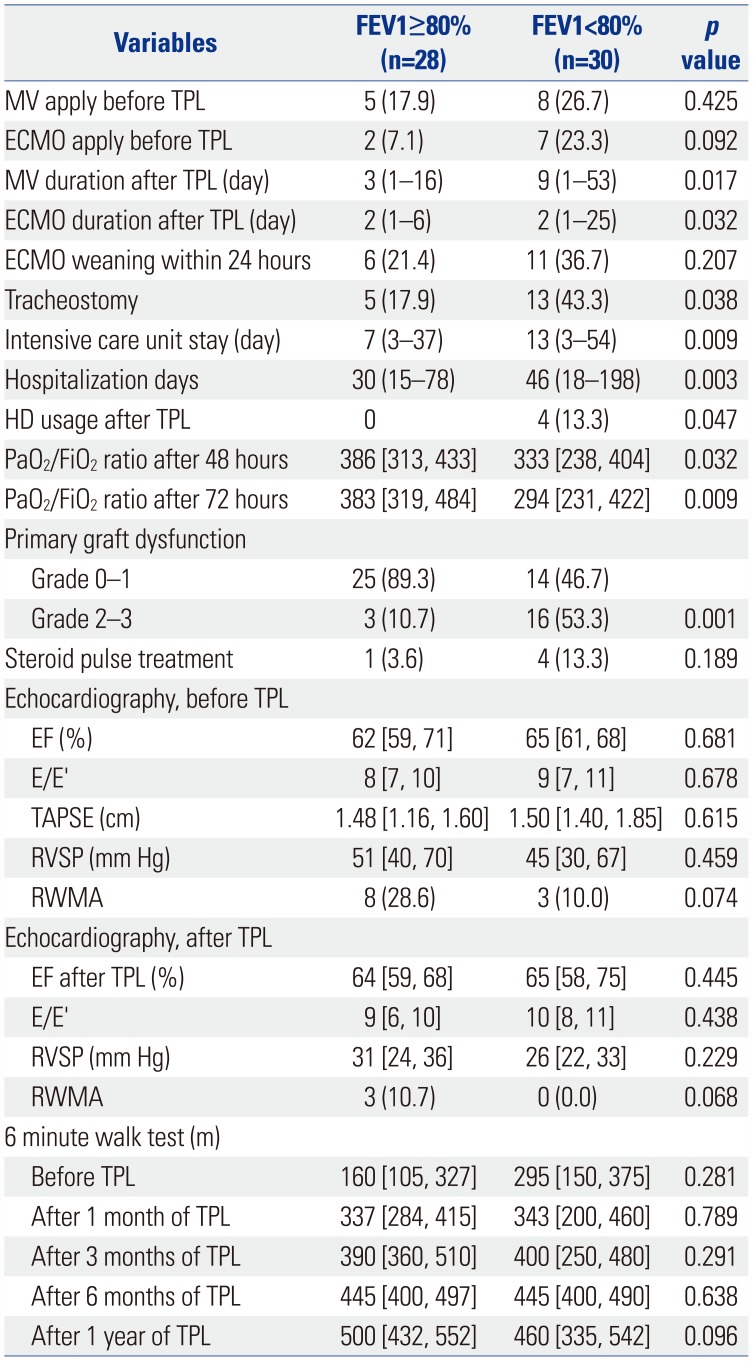
FEV1, forced expiratory volume in 1 second; MV, mechanical ventilation; TPL, transplantation; ECMO, extracorporeal membrane oxygenation; HD, hemodialysis; PaO2, arterial partial pressure of oxygen; FiO2, fraction of inspired oxygen; EF, ejection fraction; E/E′, the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E′); TAPSE, tricuspid annular plane systolic excursion; RVSP, right ventricular systolic pressure; RWMA, regional wall motion abnormalities.
Data are presented as numbers (percentage), median (range), or median [interquartile range].
Table 4
Univariate Analysis of Variables Related with Transplantation Surgery
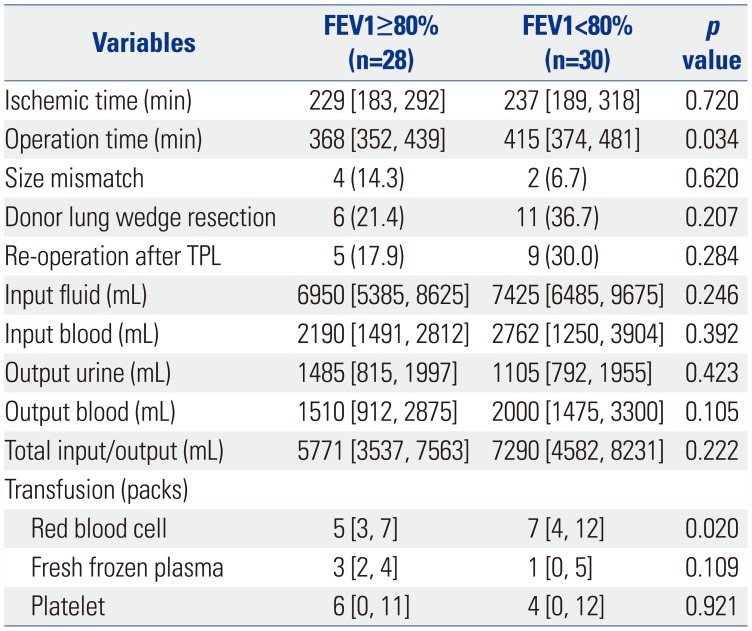
Table 5
Univariate Analysis of Variables Related with Donors
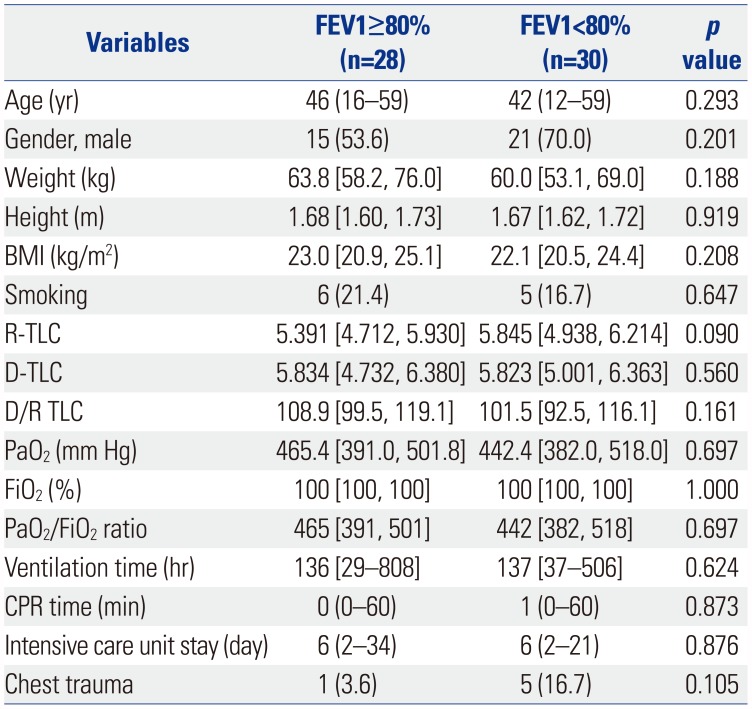
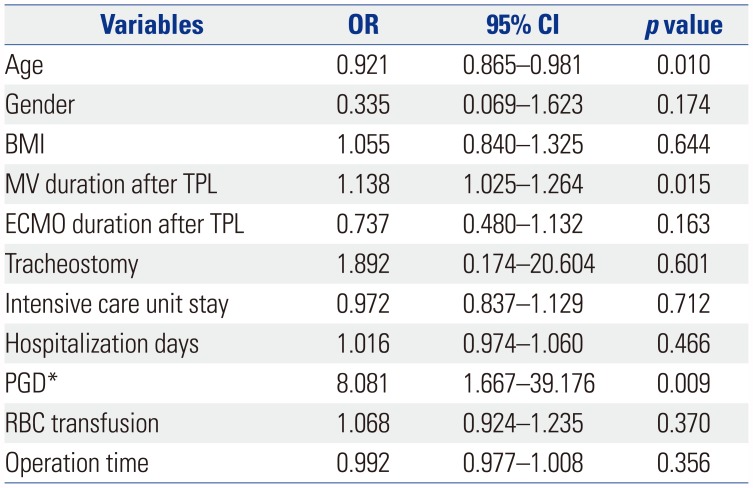
Table 6
Multivariable Analysis of Factors Affecting Pulmonary Function Recovery




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download