Abstract
Purpose
This study was designed as a meta-analysis of randomized controlled trials (RCTs) that included the comparison of palonosetron and ramosetron for postoperative nausea and vomiting (PONV) prophylaxis.
Materials and Methods
A systematic search was conducted for the PubMed, EMBASE, Web of Science, CENTRAL, KoreaMed, and Google Scholar databases (PROSPERO protocol number CRD42015026009). Primary outcomes were the incidences of postoperative nausea (PON) and postoperative vomiting (POV) during the first 48 hrs after surgery. The total 48-hr period was further analyzed in time epochs of 0–6 hrs (early), 6–24 hrs (late), and 24–48 hrs (delayed). Subgroup analyses according to number of risk factors, sex, and type of surgery were also performed.
Results
Eleven studies including 1373 patients were analyzed. There was no difference in PON or POV between the two drugs for the total 48-hr period after surgery. However, palonosetron was more effective in preventing POV during the delayed period overall [relative risk (RR), 0.59; 95% confidence interval (CI), 0.39 to 0.89; p=0.013], as well as after subgroup analyses for females and laparoscopies (RR, 0.56; 95% CI, 0.36 to 0.86; p=0.009 and RR, 0.46; 95% CI, 0.23 to 0.94; p=0.033). Subgroup analysis for spine surgery showed that ramosetron was more effective in reducing POV during the total 48-hr (RR, 3.34; 95% CI, 1.46 to 7.63; p=0.004) and early periods (RR, 8.47; 95% CI, 1.57 to 45.72; p=0.013).
Defined as any nausea, retching, or vomiting occurring during the first 24 to 48 hours after surgery, postoperative nausea and vomiting (PONV) continues to be a significant clinical problem, despite decades of searching for more effective antiemetic drugs and strategies. The major classes of drugs used for PONV prophylaxis include serotonin antagonists, neurokinin-1 receptor antagonists, corticosteroids, butyrophenones, and phenothiazines.1 One of the most widely used among these drugs are serotonin antagonists, namely 5-hydroxytryptamine 3 (5HT3) receptor antagonists.2 Ramosetron is a relatively newer 5-HT3 antagonist with a higher affinity and more prolonged activity than previously developed drugs, such as ondansetron and granisetron. Even after excluding the retracted papers by Mihara, et al.,3 ramosetron was found to have a significant effect in preventing PONV, compared to placebo, and also the ability to prevent early and late postoperative vomiting (POV) better than ondansetron. More recently, a second generation 5-HT3 antagonist, palonosetron, with greater receptor binding affinity and a half-life as long as 40 hours, has gained popularity as an effective anti-emetic. Palonosetron was reported to provide better prophylaxis of early and late postoperative nausea (PON) and late POV, compared to ondansetron.4
According to the most recent consensus guidelines for PONV management that were published in 2014,1 we can employ either a risk-adapted approach (e.g., no prevention in low-risk patients) or a general multimodal prevention strategy for PONV prevention. Regardless of the type of strategy we choose, however, optimizing a PONV prevention protocol should be based on clinical evidence of the characteristics and comparative efficacies of available drugs. While both palonosetron and ramosetron have been reported to be superior to ondansetron for PONV prevention,34 whether one has better efficacy over the other drug is not clear. In light of the increasing importance of postdischarge nausea and vomiting (PDNV),5 an antiemetic that is effective for durations longer than 24 hours cannot be ignored. This meta-analysis was performed to investigate whether palonosetron 0.075 mg is superior to ramosetron 0.3 mg for the prevention of PONV during the first 48 hrs after surgery in adult patients.
This systematic review and meta-analysis included randomized controlled trials (RCTs) that included the comparison of palonosetron and ramosetron for prevention of PONV after surgery under either general or regional anesthesia. Our study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,6 and our protocol was registered with PROSPERO (CRD420150 26009).
Two authors (SHP and MSK) independently searched PubMed, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), KoreaMed, and Google Scholar databases up to September 29, 2016 without language limitations for relevant clinical studies. The search strategy consisted of a combination of the following free text words and Medical Subject Headings (MeSH) terms: “palonosetron”, “ramosetron”, “postoperative”, “postanesthetic”, “postanaesthetic”, “nausea”, “vomiting”, “emesis”, and “retching”. The following terms were used for our search in PubMed: ((((ramosetron) AND (palonosetron)) AND ((((nausea) OR vomiting) OR emesis) OR retching)) AND (((((PONV) OR postoperative) OR postanesthetic) OR postanaesthetic) OR surgical). Any disagreements over inclusion or exclusion of a study were resolved by the third author (YSC).
Studies using standard doses of palonosetron 0.075 mg and ramosetron 0.3 mg without other adjuncts, such as dexamethasone, for prevention of PONV were included in the systematic review. Study participants were all 18 years or older, undergoing any type of elective surgery involving general or regional anesthesia, and administered either palonosetron or ramosetron intravenously.
Primary outcome measures were incidence of PON or POV during the first 48 hrs after surgery. The first 24 hrs after surgery were divided and defined as two different periods: the early and late periods (e.g., 0–6 and 6–24 hrs). However, if the first 24 hrs were divided into three periods, such as 0–2, 2–6, and 6–24 hrs, the second period was defined as the early period, and the third as the late period.4 Also, studies that divided the first 24 hrs into three periods, such that the durations of the first and second period were relatively too short [e.g., 0 to arrival at post-anesthesia care unit (PACU), from arrival to discharge from PACU, from discharge from PACU to 24 hr], the first two periods were combined and defined as the early period. The following 24 hrs after surgery (24–48 hrs) were defined as the delayed period, when presented by the authors of each study. The entire study period of up to 48 hrs in each study were defined and analyzed as the total period. Study periods beyond 48 hrs (e.g., 48 to 72 hrs) were not included in the total period. PON and POV were evaluated only as categorical data (i.e., yes/no) and not severity. In studies that presented their data on severity scoring scales, the presence of PON or POV was defined as any score above 0. Secondary outcomes were the proportion of participants that showed complete response (CR), the proportion of participants that received rescue antiemetics during each study period, and the incidence of common adverse effects of the study drugs.
Nausea was defined as a subjectively unpleasant sensation associated with awareness of the urge to vomit, whereas vomiting was defined as either vomiting (forceful expulsion of gastric contents from the mouth) or retching (labored, spasmodic, rhythmic contraction of the respiratory muscles without the expulsion of gastric contents).7 A CR to palonosetron or ramosetron was defined as the absence of any nausea or vomiting.
From the final selected studies, the following data were extracted: name of first author, year of publication, country of origin, number and characteristics of enrolled participants, type of surgery and anesthesia, treatment regimen used for antiemetics and analgesics, and primary and secondary outcomes of the present systematic review. When relevant data were presented in graphical results or were missing from the manuscript, the authors were contacted via e-mail.
When the values were presented as median and total range or an interquartile range (IQR) of values, the mean value was estimated from the devised formula using values of the median, low and high end of the range for samples less than 25, and the median value itself was regarded as the mean value for samples more than 25. The standard deviation was estimated from the devised formula using values of the median, low and high end of the range for samples less than 15, as the range/4 for samples from 15 to 70 and as the range/6 for samples greater than 70. When only an IQR was provided from selected articles, the standard deviation was calculated using IQR/1.35.89
Risk of bias in individual studies was evaluated by two authors (SS and JHP) according to the Cochrane Collaboration's tool consisting of selection, performance, detection, attrition, and reporting and other bias.10 The bias was graded as ‘low risk,’ ‘high risk,’ or ‘unclear.’ Inter-rater agreement level on risk of bias was assessed by running Cohen's kappa and following the guidelines from Landis and Koch.11
Stata software (Version 14.0; Stata Corporation, College Station, TX, USA) was used to perform the meta-analyses. All statistical outcomes were presented with 95% confidence intervals (CIs). For dichotomous variables, we calculated the relative risk (RR) at the individual trial level and the pooled RR using the Mantel-Haenszel method in a fixed effects model or the DerSimonian-Laird method in a random-effects model. The presence of heterogeneity was evaluated with the I2 test. When an I2 value more than 50% was observed, heterogeneity was regarded as substantial, and the random effect model was applied. Otherwise, the fixed-effect model was used. Subgroup analyses were performed based on number of risk factors, patient sex, and type of surgery (laparoscopy or spine surgery). Visual observation of funnel plots and Egger's linear regression test were performed to assess the possibility of publication bias for outcomes obtained from more than three studies. An asymmetric funnel plot and a p value less than 0.10 on Egger's test indicated the possible presence of publication bias.
Our initial electronic database search yielded 69 citations. Of these, 35 were found to be duplications and were excluded. Of the remaining studies, 22 were non-relevant to our meta-analysis, and one was a conference abstract. The full text articles were retrieved for the remaining 11 studies and were found to fulfill our criteria for systematic review and meta-analysis (Fig. 1).1213141516171819202122
A total of 1373 participants were included in the studies analyzed in this systematic review, of which 685 patients received palonosetron 0.075 mg and 688 patients received ramosetron 0.3 mg intravenously (Table 1). Inhalation anesthesia was performed in 10 studies13141516171819202122 and one study used spinal anesthesia.12 Four studies gave antiemetics before induction of anesthesia,13161720 while another four studies gave them at the end of surgery.14151819 The two antiemetics were administered at different time points in the study by Yoon, et al.,21 in which palonosetron was administered just after anesthesia induction and ramosetron at the end of surgery. Antiemetics were administered twice in the study by Song, et al.,22 which gave the study drugs at the end of surgery and at 24 hrs after surgery. One study involving cesarean section gave the study drugs after clamping of the umbilical cord to prevent any possible transfer to the fetus.12 Nine of the studies included the 24–48 hr period in their study,121314151617182122 which was defined as the delayed period in the present review. Three studies divided the first 24 hours after surgery into three periods,13,19,20 and therefore, the second periods of these studies were defined as the early period and data of the first period was not included in the analysis. Three other studies included data for postoperative 48–72 hr141718 which were not analyzed in the present report. The manuscript by Yoon, et al.21 was published in Korean, with an abstract available in English. All other papers were in English.
The risks of bias are shown in Table 2. All of the studies were graded as unclear or low risk regarding random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other possible bias. Only the study by Lee, et al.15 was graded as high risk in blinding of outcome assessment. Four studies13161721 lacking description of any conflict of interest were graded as unclear in other bias. Cohen's kappa value was 0.79 (95% CI, 0.66 to 0.93; p<0.001), suggesting substantial agreement between the two raters that evaluated risk of bias.
The results of pooled analyses for PON are summarized in Table 3. There was no difference in PON between patients receiving palonosetron and ramosetron during the total (RR 1.09; 95% CI, 0.97 to 1.22; I2=16.8%; p=0.135), early (RR, 0.89; 95% CI, 0.61 to 1.31; I2=61.5%; p=0.561), late (RR, 0.86; 95% CI, 0.64 to 1.16; I2=59.9%; p=0.322), and delayed (RR, 1.04; 95% CI, 0.74 to 1.47; I2=50.3%; p=0.810) periods. Considerable heterogeneity was found in the data for early and late PON. No difference was seen in PON between groups in subgroup analyses done for risk factors, sex, or type of surgery.
Table 4 shows pooled analyses for POV. There were three studies that did not provide POV data for the three separate time periods.131920 Swaika, et al.19 and Yatoo, et al.20 did not present delayed POV data, while Kim, et al.13 presented POV data of the total period only. The studies by Piplai, et al.,17 Chattopadhyay and Goswami,12 and Lee, et al.15 all presented POV data for the early, late, and delayed periods but not for the total study period. Because the study by Kim, et al.13 was the only study done in high risk patients, subgroup analyses according to risk factors were not done for early, late, and delayed POV. During the total study period there was no difference in POV between palonosetron and ramosetron overall or in subgroup analyses for risk factors, sex, and laparoscopies. However, subgroup analysis for spine surgery showed that ramosetron was significantly more effective than palonosetron in reducing POV during the total study period (RR, 3.34; 95% CI, 1.46 to 7.63; I2= 0.0%; p=0.004). While there was no difference in POV between the two groups during the late period, significant differences in POV were observed for the early and delayed periods. Palonosetron was found to be significantly more effective than ramosetron in preventing POV in females and laparoscopies during the early (RR, 0.60; 95% CI, 0.38 to 0.95; I2=0.0%; p=0.028 and RR, 0.54; 95% CI, 0.33 to 0.89; I2=0.0%; p=0.015) and delayed (RR, 0.56; 95% CI, 0.36 to 0.86; I2=0.0%; p=0.009 and RR, 0.46; 95% CI, 0.23 to 0.94; I2=0.0%; p=0.033) periods after surgery (Figs. 2 and 3). Palonosetron was also found to be more effective in the overall analyses for the delayed period (RR, 0.59; 95% CI, 0.39 to 0.89; I2=0.0%; p=0.013). Interestingly, subgroup analysis for spine surgery in the early period showed that ramosetron was significantly better than palonosetron in reducing POV, with a high RR (RR, 8.47; 95% CI, 1.57 to 45.72; I2=0.0%; p=0.013) (Fig. 2).
The pooled analyses from studies providing data on CR rates are shown in Table 5. There was no overall difference between palonosetron and ramosetron on CR over the total study period. However, when subgroup analyses was conducted after excluding one RCT that was conducted only in patients at high risk of PONV,13 ramosetron was found to result in higher CR than palonosetron (RR, 0.86; 95% CI, 0.76 to 0.98; I2=19.8%; p=0.023). In the eight studies that presented CR data for three different time periods,1214161718202122 no difference was found between palonosetron and ramosetron in the overall analysis, as well as in subgroup analyses for sex and for type of surgery. The study by Kim, et al.,13 which was done only in high risk patients, did not provide CR data for separate time periods and was thus not included in the subgroup analyses for early, late, and delayed periods.
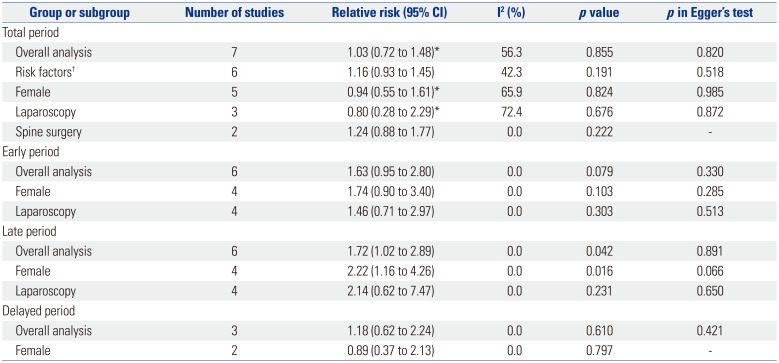
Results of pooled analyses for rescue antiemetics are shown in Table 6, and no difference was found between palonosetron and ramosetron with regard to the need for rescue antiemetics during the total postoperative period. This was also true for the early and delayed periods. However, patients that received palonosetron were found to require more rescue antiemetics than those that received ramosetron (RR, 1.72; 95% CI, 1.02 to 2.89; I2=0.0%; p=0.042) during the late period. This difference was also seen in female patients during the late period (RR, 2.22; 95% CI, 1.16 to 4.26; I2=0.0%; p=0.016). Subgroup analysis for risk factors was not done for early, late, and delayed periods, as the study performed in high risk patients13 did not provide data for rescue antiemetics for separate time periods.
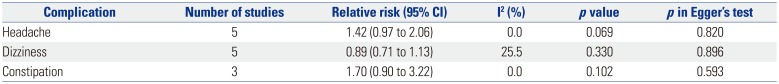
The results of pooled analyses for complications are shown in Table 7. Among the various side effects that were studied in each study, we were able to analyze the data reported for headache, dizziness, and constipation during the total study period, and found no difference between palonosetron and ramosetron for all three complications.
There have been recent previous meta-analyses comparing either palonosetron4 or ramosetron323 with ondansetron, but a direct comparison between each other has not yet been made. Xiong, et al.4 reported that palonosetron provides better prophylaxis against early PON (0–6 hr), late PON (6–24 hr), and late POV (6–24 hr), compared to ondansetron. However, this analysis did not provide data for time periods after 24 hrs. Gao, et al.23 found ramosetron to be more effective than ondansetron for prophylaxis of POV at 0–24 hrs with fewer side effects, but not at 24–48 hrs. We compared palonosetron with ramosetron up to 48 hrs after surgery in the present meta-analysis, and although it is difficult to draw a definite conclusion with regards to the superiority of one drug over the other, it seems that palonosetron is more effective that ramosetron for the prevention of POV at postoperative 24–48 hrs. This effect was also seen in females and after laparoscopies during the first 6 hours after surgery and 24–48 hours. However, these findings were reversed in the subgroup of patients undergoing spine surgery, where ramosetron was found to be more effective in preventing POV. Also, prophylaxis with ramosetron was found to require less rescue antiemetics than palonosetron at 6–24 hours after surgery.
The concept of PONV after discharge, namely PDNV, has grown in importance. The latest guidelines for the management of PONV1 have added a new validated simplified risk score for PDNV in adults, underlining its significance. The current metaanalysis included three different periods, the last one being the “delayed period”, defined as 24 to 48 hrs after surgery. This period should be considered an important time frame, as patients will still present with significant nausea and vomiting in roughly 30%.24 The results of our study show that palonosetron was more effective in reducing POV than ramosetron during the delayed period overall, as well as in females and after laparoscopic surgery. This is probably due to the longer half-life and receptor binding characteristics of palonosetron,252627 and may imply an advantage of its use over ramosetron for patients undergoing ambulatory surgery. Palonosetron was also found to be advantageous in females and in laparoscopies during the early period, compared to ramosetron.
An interesting finding of the current analysis was the results of subgroup analysis of POV for the total period and early period. As mentioned above, palonosetron was found to be better than ramosetron in preventing POV in females and laparoscopies during the early and delayed periods. However, the results were the opposite in the subgroup analysis for spine surgery, which showed better POV prevention with ramosetron during the total and early periods. The reason of these conflicting results between types of surgery is not clear. Postoperative pain is known to prolong gastric-emptying time, which may contribute to emesis after surgery. Moreover, pain of pelvic or visceral origin has been suggested as a significant cause of PONV.28 Although palonosetron and ramosetron are both 5-HT3 receptor antagonists, palonosetron is structurally distinct from first-generation drugs and exhibits allosteric binding and positive cooperativity.29 However, whether palonosetron has greater efficacy after abdominal or pelvic surgery is not known and cannot be judged from the present analysis. With the exception of the study done by Yatoo, et al.,20 the remaining six studies done in laparoscopies131415161719 were also all conducted in females, which resulted in a great overlapping between the two subgroups. It should also be taken into account that, in contrast to the overwhelming majority of females in laparoscopy studies, males and females were enrolled at a similar ratio in spine surgery studies.
Unlike the results of POV, our analysis was not able to find any difference in PON in any of the postoperative periods or subgroups. While POV is generally assessed as a dichotomous variable of yes or no, PON can be assessed according to severity on a Visual Analogue Scale or a Likert-scale, such as mild-moderate-severe. This method of assessment is significant in that while ‘mild nausea’ may not require any rescue antiemetics, moderate to severe nausea often does and, therefore, would be more relevant to clinical practice from the patient's perspective. As the majority of the studies that were included in our present analysis presented their results as yes or no variables, our results were also analyzed and presented in the same manner. However, the lack of difference in PON between the two drugs may be partially attributable to assessing and analyzing nausea as incidence rather than on a quantitative scale.
Among the analyzed studies, one was conducted only in patients with four risk factors for PONV,13 which can be defined as high risk adult patients with an 80% risk for PONV.1 All of the patients enrolled in this study were female non-smokers with a history of motion sickness or PONV, and also received postoperative opioids.13 It is noteworthy that the significant difference in CR rates between palonosetron and ramosetron can be observed only when this aforementioned study done in high risk patients was excluded from analysis (RR, 0.86; 95% CI, 0.76 to 0.98; I2=19.8%; p=0.023) (Table 5). Although the proportion of high risk patients included in the other 10 studies are not known, it seems that the ability of a single 5-HT3 antagonist to lead to complete absence of nausea and vomiting is dampened when the percentage of high risk patients gets bigger. It is clear that the antiemetic effects of palonosetron or ramosetron, or any other drug for that matter, is not strong enough on its own when used alone in patients at high risk of PONV. Prophylaxis with combination therapy or a multimodal approach including two or more interventions is recommended in patients at high risk of PONV,1 and therefore, a single antiemetic may not have been effective enough to elicit a difference in CR rates between the two groups. Considering the latest consensus guidelines for the management of PONV,1 a more realistic clinical trial would be to study the antiemetic effects of 5-HT3 antagonists as part of a multimodal prevention method in high risk patients receiving at least one other prophylactic intervention or drug. The fact that ramosetron was found to result in a higher CR rate in a patient population with heterogenic risk for PONV is in itself inconclusive.
In terms of adverse events related to the administration palonosetron or ramosetron, four studies failed to present any relevant data,14151920 although three of these studies141520 commented that there were no differences between groups with regards to headache and dizziness. While there was a considerable difference in the adverse events that were evaluated in each study, the most commonly noted complications were headache, dizziness, and constipation. Our analyses found no difference in the incidence of these three adverse effects between palonosetron and ramosetron. However, due to the lack of data, it is difficult to definitely say that a difference in side-effect profile between palonosetron and ramosetron does not exist. None of the studies included in the present meta-analysis reported any severe cardiac adverse events, such as QT prolongation or fatal arrhythmias, which corresponds to the results of previous studies that reported the safety of palonosetron and ramosteron on the QT interval.30313233
Our meta-analysis has several limitations. Most importantly, the numbers of RCTs and patients that were analyzed in our study were relatively small. Also, the ethnicity of the patients was restricted to Indians and Asians only. Ethnicity may well influence the risk of PONV, and there have been reports pointing out certain ethnicities to be protective against PONV34 or susceptible to motion sickness,35 the latter known as an important risk factor of PONV.
As mentioned earlier, aside from one study that was conducted only in high risk patients,13 the baseline risk factors of PONV were not consistent between the included trials. Nitrous oxide is well known to increase the risk of severe PONV, and a recent study suggested that this effect is stronger in Asian patients.36 Five of the included trials used nitrous oxide with an inhalation anesthetic,1517192021 and most of the patients enrolled in these trials were Asians. Therefore, the baseline risk of PONV may have been higher in these studies, compared to an otherwise non-biased study population. Also, one study was conducted in parturients undergoing cesarean section under spinal anesthesia, the significance of which is not clear. Although the equipotent doses of palonosetron and ramosetron for PONV prevention are not known, the generally used doses are 0.075 mg and 0.3 mg, respectively. Whereas 10 of the studies included in the present analysis used these aforementioned doses for each drug as a single prophylactic injection either at the beginning or the end of surgery, the study by Song, et al.22 administered each drug twice at the end of surgery and 24 hours after surgery.
Finally, the definitions of the early and late periods varied between the included studies. However, the delayed period as defined in the current analysis was identical in all of the nine trials that evaluated this time frame, which adds weight to the results that show a significantly lower incidence of POV with palonosetron during the delayed period.
In conclusion, the present meta-analysis was not able to reveal a definite difference between palonosetron 0.075 mg and ramosetron 0.3 mg in the ability to prevent PONV. However, subgroup analyses showed that palonosetron was more effective for the prophylaxis of delayed POV, compared to ramosetron, which may be attributed to the longer half-life of palonosetron. While palonosetron seems to have an advantage over ramosetron in females and patients undergoing laparoscopic surgery, ramosetron may be more effective after spine surgery. These differences observed in subgroup analyses needs to be confirmed by further studies in order to conclude that there is an actual difference in the mechanism of action between the two anti-emetics. Also, in the light of the increasing importance and interest in delayed PONV and PONV after discharge, further studies on the prophylactic efficacy and safety of palonosetron up to postoperative 72 hrs will help refine PONV protocols for ambulatory surgery and patients at risk of prolonged PONV.
References
1. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014; 118:85–113. PMID: 24356162.
2. Wiesmann T, Kranke P, Eberhart L. Postoperative nausea and vomiting-a narrative review of pathophysiology, pharmacotherapy and clinical management strategies. Expert Opin Pharmacother. 2015; 16:1069–1077. PMID: 25866213.
3. Mihara T, Tojo K, Uchimoto K, Morita S, Goto T. Reevaluation of the effectiveness of ramosetron for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Anesth Analg. 2013; 117:329–339. PMID: 23757469.
4. Xiong C, Liu G, Ma R, Xue J, Wu A. Efficacy of palonosetron for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Can J Anaesth. 2015; 62:1268–1278. PMID: 26296300.

5. Apfel CC, Philip BK, Cakmakkaya OS, Shilling A, Shi YY, Leslie JB, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology. 2012; 117:475–486. PMID: 22846680.

6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94. PMID: 19622512.

7. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992; 77:162–184. PMID: 1609990.
8. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13. PMID: 15840177.

9. Maitra S, Baidya DK, Bhattacharjee S, Khanna P. Evaluation of igel(™) airway in children: a meta-analysis. Paediatr Anaesth. 2014; 24:1072–1079. PMID: 25041224.
10. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID: 22008217.

11. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.

12. Chattopadhyay S, Goswami S. Palonosetron versus ramosetron prophylaxis for control of postoperative nausea and vomiting after cesarean delivery under spinal anesthesia. J Obstet Gynaecol India. 2015; 65:28–33. PMID: 25737619.

13. Kim SH, Hong JY, Kim WO, Kil HK, Karm MH, Hwang JH. Palonosetron has superior prophylactic antiemetic efficacy compared with ondansetron or ramosetron in high-risk patients undergoing laparoscopic surgery: a prospective, randomized, double-blinded study. Korean J Anesthesiol. 2013; 64:517–523. PMID: 23814652.

14. Kim SH, Oh CS, Lee SJ. Efficacy of palonosetron and ramosetron on postoperative nausea and vomiting related to intravenous patient-controlled analgesia with opioids after gynecological laparoscopic surgery (double-blinded prospective randomized controlled trial). J Anesth. 2015; 29:585–592. PMID: 25735497.

15. Lee WS, Lee KB, Lim S, Chang YG. Comparison of palonosetron, granisetron, and ramosetron for the prevention of postoperative nausea and vomiting after laparoscopic gynecologic surgery: a prospective randomized trial. BMC Anesthesiol. 2015; 15:121. PMID: 26335706.

16. Park SK, Cho EJ, Kang SH, Lee YJ, Kim DA. A randomized, doubleblind study to evaluate the efficacy of ramosetron and palonosetron for prevention of postoperative nausea and vomiting after gynecological laparoscopic surgery. Korean J Anesthesiol. 2013; 64:133–137. PMID: 23459596.

17. Piplai G, Chakrabarty I, Mukhopadhyay M, Karmakar M, Sarkar S, Bhattacharjee PD. A comparative study between palonosetron and ramosetron to prevent postoperative nausea and vomiting after laparoscopic cholecystectomy. Int Res J Pharm Pharmacol. 2012; 2:193–197.
18. Roh GU, Yang SY, Shim JK, Kwak YL. Efficacy of palonosetron versus ramosetron on preventing opioid-based analgesia-related nausea and vomiting after lumbar spinal surgery: a prospective, randomized, and double-blind trial. Spine (Phila Pa 1976). 2014; 39:E543–E549. PMID: 24480956.
19. Swaika S, Pal A, Chatterjee S, Saha D, Dawar N. Ondansetron, ramosetron, or palonosetron: which is a better choice of antiemetic to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy? Anesth Essays Res. 2011; 5:182–186. PMID: 25885385.

20. Yatoo FA, Mahotra KK, Mehta N, Gupta KC, Sidhra J. A comparative study of granisetron, ramosetron and palonosetron as antiemetics in prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgeries. J Evolution Med Dent Sci. 2016; 5:1229–1234.
21. Yoon HJ, Jee YS, Kim YD. Comparison of the efficacy of ramosetron and palonosetron for prevention of postoperative nausea and vomiting in patients undergoing gynecologic oncology surgery. Anesth Pain Med. 2016; 11:264–268.

22. Song JW, Shim JK, Choi SH, Soh S, Jang J, Kwak YL. Comparison of ramosetron and palonosetron for preventing nausea and vomiting after spinal surgery: association with ABCB1 polymorphisms. J Neurosurg Anesthesiol. 2016; 8. 25. [Epub]. DOI: 10.1097/ANA.0000000000000361.

23. Gao C, Li B, Xu L, Lv F, Cao G, Wang H, et al. Efficacy and safety of ramosetron versus ondansetron for postoperative nausea and vomiting after general anesthesia: a meta-analysis of randomized clinical trials. Drug Des Devel Ther. 2015; 9:2343–2350.

24. Odom-Forren J, Jalota L, Moser DK, Lennie TA, Hall LA, Holtman J, et al. Incidence and predictors of postdischarge nausea and vomiting in a 7-day population. J Clin Anesth. 2013; 25:551–559. PMID: 23988801.

25. Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, et al. The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther. 2010; 335:362–368. PMID: 20724484.

26. Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010; 626:193–199. PMID: 19836386.

27. Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008; 107:469–478. PMID: 18633025.

28. Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000; 59:213–243. PMID: 10730546.

29. Yang LP, Scott LJ. Palonosetron: in the prevention of nausea and vomiting. Drugs. 2009; 69:2257–2278. PMID: 19852528.
30. Kim SH, Lee SM, Kim YK, Park SY, Lee JH, Cho SH, et al. Effects of prophylactic ramosetron and ondansetron on corrected QT interval during general anesthesia. J Clin Anesth. 2014; 26:511–516. PMID: 25439413.

31. Kwon MJ, Han J, Seo JH, Song K, Jeong HM, Choi JS, et al. CD24 overexpression is associated with poor prognosis in luminal A and triple-negative breast cancer. PLoS One. 2015; 10:e0139112. PMID: 26444008.

32. Kim HJ, Lee HC, Jung YS, Lee J, Min JJ, Hong DM, et al. Effect of palonosetron on the QTc interval in patients undergoing sevoflurane anaesthesia. Br J Anaesth. 2014; 112:460–468. PMID: 24129597.

33. Min JJ, Kim HJ, Jung SY, Kim BG, Kwon K, Jung HJ, et al. Effects of palonosetron on perioperative cardiovascular complications in patients undergoing noncardiac surgery with general anesthesia: a retrospective cohort study. Clin Pharmacol Ther. 2015; 98:96–106. PMID: 25786663.

34. Rodseth RN, Gopalan PD, Cassimjee HM, Goga S. Reduced incidence of postoperative nausea and vomiting in black South Africans and its utility for a modified risk scoring system. Anesth Analg. 2010; 110:1591–1594. PMID: 20385613.

35. Stern RM, Hu S, Uijtdehaage SH, Muth ER, Xu LH, Koch KL. Asian hypersusceptibility to motion sickness. Hum Hered. 1996; 46:7–14. PMID: 8825456.

36. Myles PS, Chan MT, Kasza J, Paech MJ, Leslie K, Peyton PJ, et al. Severe nausea and vomiting in the rvaluation of nitrous oxide in the gas mixture for anesthesia II trial. Anesthesiology. 2016; 124:1032–1040. PMID: 26904965.
Fig. 2
Forest plot for postoperative vomiting during the early period according to type of surgery. Results of subgroup analysis for laparoscopies, spine surgery and cesarean section (neither a laparoscopy or spine surgery) are shown. RR, relative risk; CI, confidence interval.
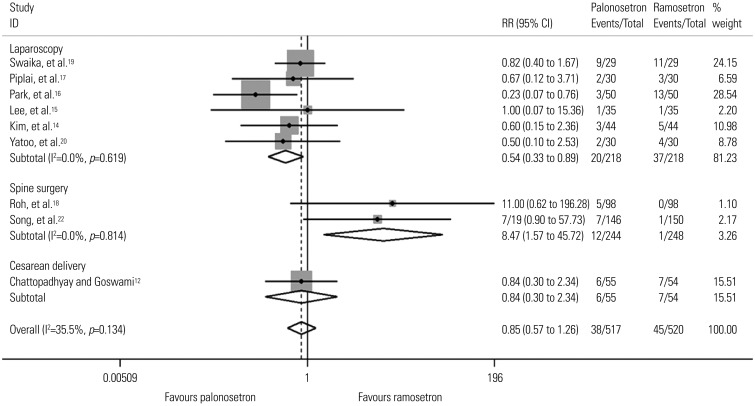
Fig. 3
Forest plot for postoperative vomiting during the delayed period according to gender. Results of subgroup analysis for studies done only in females and those conducted in both genders are shown. RR, relative risk; CI, confidence interval.
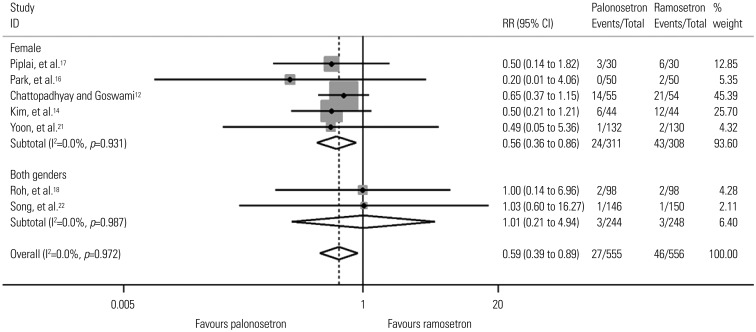
Table 1
Summary of the Included Studies
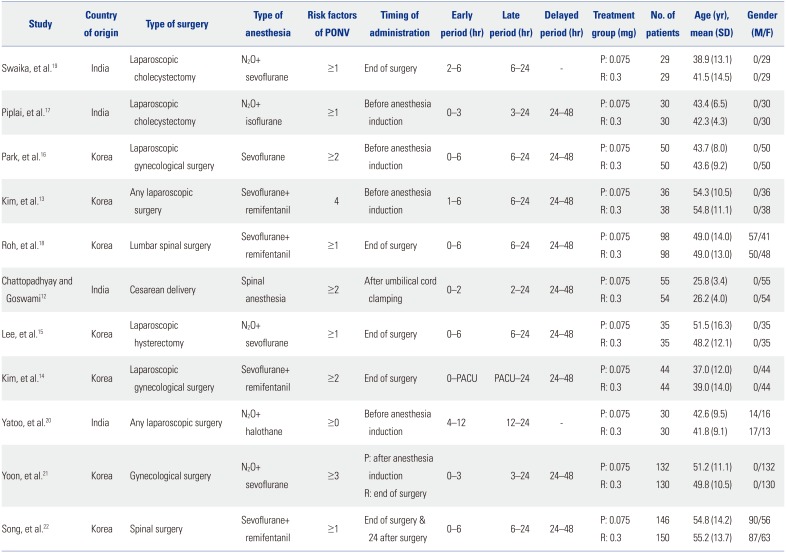
Table 2
Summary of the Risk of Bias
| Study | Random sequence generation | Allocation concealment | Blinding of participant and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Swaika, et al.19 | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear | Low risk |
| Piplai, et al.17 | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Park, et al.16 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Kim, et al.13 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Roh, et al.18 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Chattopadhyay and Goswami12 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lee, et al.15 | Low risk | Unclear | Unclear | High risk | Low risk | Low risk | Low risk |
| Kim, et al.14 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Yatoo, et al.20 | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk |
| Yoon, et al.21 | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk | Unclear |
| Song, et al.22 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Table 3
Postoperative Nausea
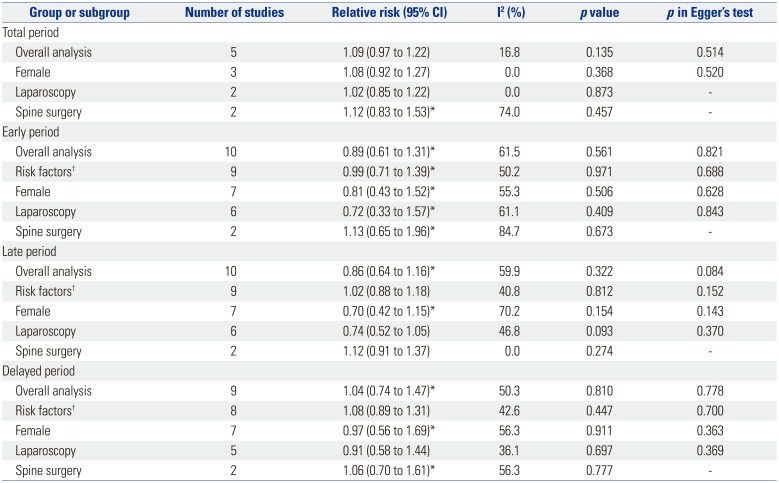
Table 4
Postoperative Vomiting
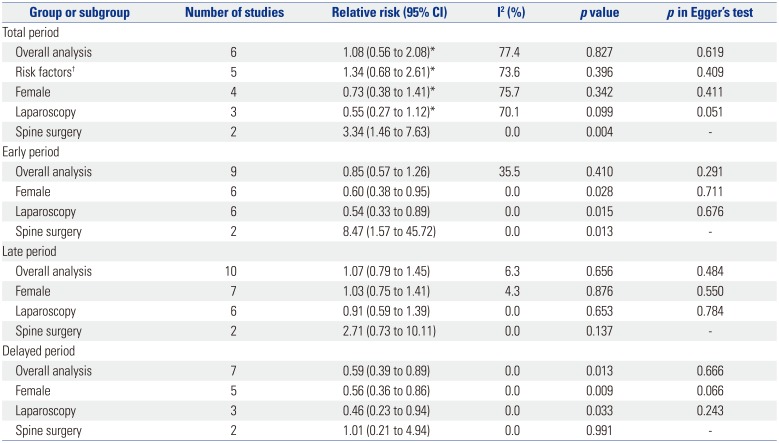
Table 5
Complete Response
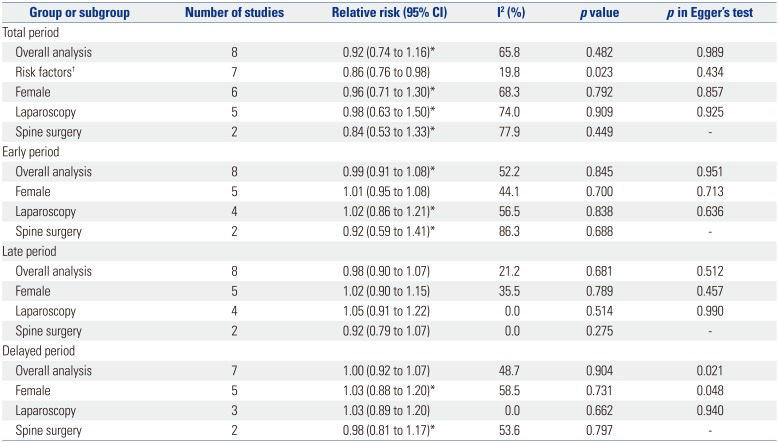
Table 6
Rescue Antiemetics




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download