Abstract
Purpose
Infliximab is currently used for the treatment of active Crohn's disease (CD). We aimed to assess the efficacy and safety of infliximab therapy and to determine the predictors of response in Korean patients with CD.
Materials and Methods
A total of 317 patients who received at least one infliximab infusion for active luminal CD (n=198) and fistulizing CD (n=86) or both (n=33) were reviewed retrospectively in 29 Korean referral centers. Clinical outcomes of induction and maintenance therapy with infliximab, predictors of response, and adverse events were evaluated.
Results
In patients with luminal CD, the rates of clinical response and remission at week 14 were 89.2% and 60.0%, respectively. Male gender and isolated colonic disease were associated with higher remission rates at week 14. In week-14 responders, the probabilities of sustained response and remission were 96.2% and 93.3% at week 30 and 88.0% and 77.0% at week 54, respectively. In patients with fistulizing CD, clinical response and remission were observed in 85.0% and 56.2% of patients, respectively, at week 14. In week-14 responders, the probabilities of sustained response and remission were 94.0% and 97.1%, respectively, at both week 30 and week 54. Thirty-nine patients (12.3%) experienced adverse events related to infliximab. Serious adverse events developed in 19 (6.0%) patients including seven cases of active pulmonary tuberculosis.
Crohn's disease (CD) is a chronic disorder that causes inflammation in any location of the gastrointestinal (GI) tract, although it is most commonly in the end of the small bowel and proximal large bowel.12 Patients with CD suffer from GI symptoms, such as abdominal pain and diarrhea, as well as disease-related complications, including stricture and fistula.12 Fistulas are common manifestations in CD due to its transmural nature. A variety of proteases and matrix metalloproteinases are released by immune activation, and these enzymes provoke tissue destruction, sinus tract formation, and ultimately penetration into adjacent tissues.3
Conventional medical therapies, such as 5-aminosalicylates, corticosteroids, and immunomodulators (IMMs) are effective in patients with CD; however, many patients continue to suffer from disabling conditions due to insufficient responses or side effects of these drugs.456 The introduction of infliximab, an antibody against tumor necrosis factor (TNF)-α, has greatly improved treatment efficacy in patients with CD. Infliximab is a chimeric immunoglobulin G 1 monoclonal antibody that neutralizes the biologic activity of TNF-α, which plays a central role in the pathogenesis of CD.78 In many studies, infliximab was effective in the induction and maintenance therapy of moderate to severely active CD in patients who had not responded to treatments with a corticosteroid or IMMs. In addition, infliximab has been demonstrated to effectively induce and maintain a clinical response in patients with fistulizing CD and to induce mucosal healing in patients with active luminal CD.1910 Treatment with infliximab is generally well tolerated. However, infliximab infusions can be associated with several adverse events, such as acute and delayed hypersensitivity (serum sickness-like reactions), infusion reactions, infectious complications, and hepatosplenic T-cell lymphomas.1112
To date, most studies that have evaluated the efficacy and safety of infliximab have been conducted in patients in Western countries who have different genetic, ethnic, and environmental backgrounds from Asian patients.13 There are limited data regarding the efficacy and safety of infliximab for Asian CD patients. Moreover, many of the Western studies were randomized controlled trials (RCTs) that might not have properly investigated CD patients encountered during routine clinical practice. In fact, many patients with CD evaluated in an outpatient clinic may not qualify for enrollment in an RCT of biological reagents.14 Use of infliximab is rapidly increasing with the accompanying increasing incidence of CD in Korea, and there is a specific concern about reactivation of tuberculosis (TB), as the prevalence of latent and active TB remains high.1516 Therefore, we aimed to investigate the efficacy and safety of infliximab therapy in Korean patients with luminal and/or fistulizing CD and to identify clinical predictors of response to the therapy.
This nationwide multicenter study was carried out at 29 referral hospitals in South Korea. We retrospectively reviewed the medical records of CD patients with refractory luminal and/or fistulizing disease who received at least one infliximab (Remicade®) infusion from 2002 to 2011. At each site, one member of the Korean Association for the Study of Intestinal Diseases was responsible for data collection.
Diagnosis of CD was based on standard clinical, radiologic, endoscopic, and histopathologic criteria which in turn were based on the Lennard-Jones criteria.17 Infliximab therapy was indicated for the treatment of moderate-to-severe luminal and/or fistulizing CD in patients who had not responded to aminosalicylates, corticosteroids, or IMMs.19 All patients received 5 mg/kg infliximab intravenously at weeks 0, 2, and 6 for induction therapy and then every 8 weeks for maintenance therapy if the patients' symptoms improved with induction therapy. If the response to maintenance therapy using infliximab had been inadequate, the dose was increased to 10 mg/kg.
For all eligible patients, we collected demographic data, baseline characteristics, previous and concomitant treatments, indication for infliximab, dose and number of infusions, clinical outcomes, and adverse events of infliximab treatment, particularly those associated with TB. We used the definitions of steroid dependency, steroid refractoriness, and immunomodulator refractoriness described by the European Crohn's and Colitis Organization consensus.2 The study protocol was approved by the Institutional Review Boards at all participating sites.
For the evaluation of disease activity and the response to infliximab therapy, Crohn's Disease Activity Index (CDAI) values were collected at 0, 2, 6, 14, 30, and 54 weeks and at the last follow-up visit after the first infusion of infliximab for luminal CD, and the status of fistula was determined at 0, 14, 30, and 54 weeks and at the last follow-up visit for fistulizing CD.
Definitions of clinical response and remission were adopted from the ACCENT-I and II trials.1819 In luminal CD, clinical response to treatment was defined as a decrease in CDAI score of 70 points or more from the baseline value and at least a 25% reduction in the total CDAI score, and complete response (remission) was defined as a CDAI score of less than 150. In fistulizing CD, clinical response to treatment was defined as a decrease of 50% or more in the number of fistulas and draining discharge, and complete response (remission) was defined as a complete closure of fistulas on physical examination and/or imaging studies. An external fistula was considered to be closed if no discharge was drained despite gentle finger compression. Patients with partial response were defined as those who had clinical response but did not achieve remission. Initial response and remission rates were evaluated at 2, 6, and 14 weeks for luminal CD and at 14 weeks for fistulizing CD. The estimated rates of sustained clinical response and remission were determined at weeks 30 and 54 among patients who had clinical response or remission at week 14. Loss of response or remission was defined by a CDAI score or fistula status that did not meet the response or remission criteria at weeks 30 and 54.
Factors that might affect clinical remission at week 14 and sustained clinical remission at weeks 30 and 54 were evaluated as follows: sex, age at diagnosis, age at first infusion of infliximab, time from diagnosis to first infusion, body mass index, smoking, disease location, concomitant medications, and laboratory and colonoscopic findings.
Mucosal healing was defined as the absence of mucosal ulceration in all segments examined via ileocolonoscopy. Serious adverse events were defined as those that were life threatening, required hospitalization, or led to significant disability or incapacity.20
All continuous data are expressed as mean±standard deviation. We used Student's t-test for continuous variables and the chis-quare test for discrete variables. Univariate and multivariate logistic regression analyses were used to identify predictors of complete response at week 14. A Kaplan-Meier analysis was used to estimate sustained response and remission rates at weeks 30 and 54, and a log-rank test was used to identify predictors associated with sustained clinical remission. Patients with sustained clinical response or remission were censored at the last clinical visit. All p values were two-tailed, and p values of <0.05 were considered to be statistically significant. Statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA).
A total of 317 CD patients were included in this study, and data were collected from September 2009 through September 2011. All of the patients received at least one infliximab infusion for the treatment of CD between 2002 and 2011, with a mean follow-up of 23.2±18.8 months from the first infusion. Mean ages at diagnosis and at first treatment with infliximab for CD were 25.9±10.2 (range 12–79) and 29.5±10.8 (range 14–79) years, respectively. Regarding past medical history, 17 patients had a history of active TB. A tuberculosis skin test (TST), interferon gamma releasing assay (IGRA), or both tests were performed in 183 (57.7%), 100 (31.5%), and 56 (17.7%) patients, respectively, for latent TB infection (LTBI), and positive findings of TST, IGRA, and both tests were observed in 18 (9.8%), 11 (11%), and one (1.8%) patient, respectively. A simple chest X-ray was performed in all patients before infliximab infusion, and five patients had TB scar changes. Patients who had at least one positive finding among TB scars on chest X-ray, TST, and IGRA were considered to have LTBI (n=33). Among these 33 patients, 18 patients had received TB prophylaxis. Six patients had a family history of inflammatory bowel disease (CD: 5; ulcerative colitis: 1). In colonoscopic findings, ileocolonic ulcers (excluding aphthous ulcers) were observed in 79.7% (192 of 241), and a cobblestone appearance was observed in 37.3% (90 of 241). The baseline characteristics of the patients are summarized in Table 1.
The indication of infliximab therapy was as follows: 198 patients (62.5%) were treated for active luminal CD, 86 patients (27.1%) for fistulizing CD, and 33 patients (10.4%) for both active luminal and fistulizing CD. Among the patients with active luminal CD, 56 (28.3%) had steroid dependency, 30 (15.2%) had steroid refractoriness, and 98 (49.5%) had IMM refractoriness. Among 119 patients with fistulas, 99 (83.2%) had external fistulas, and 28 (23.5%) had internal fistulas. The median number of infliximab infusions was eight, ranging from one to 31. The standard dose of 5 mg/kg infliximab was administered and maintained in most cases. During infliximab therapy, a dose increase was applied in eight patients (2.5%) and an interval decrease in two (0.6%). One hundred thirty-three (42%) patients discontinued therapy due to primary non-response (n=26), loss of response (n=23), intolerable adverse events (n=12), follow-up loss (n=16), economic difficulties (n=12), disease remission (n=27), or other reasons (n=17). A flow chart of the study population for each analysis is presented in Fig. 1.
After the first infusion of infliximab, treatment efficacy was assessed in 227 patients at week 2, 206 patients at week 6, and 185 patients at week 14 among the 231 patients who were diagnosed with luminal CD or both luminal and fistulizing CD. Clinical response and remission were observed in 163 (71.8%) and 89 (39.2%) patients at week 2, in 177 (85.9%) and 110 (53.4%) at week 6, and in 165 (89.2%) and 111 (60.0%) at week 14, respectively (Fig. 2). CDAI scores at weeks 2, 6, and 14 decreased from the baseline value of 295.0±82.9 to 150.3±72.0, 132.1±70.2, and 134.1±84.1 at each time point, respectively. An analysis to identify factors related to clinical remission at week 2 showed a significantly higher likelihood of remission in patients whose first infusion of infliximab was less than 3 years from diagnosis than in those whose first infusion was greater than 3 years (47.1% vs. 30.6%, p=0.011); however, no other factors were associated with the remission rate. There was no factor associated with the remission rate at week 6. On analysis of the factors related to clinical remission at week 14, male gender and isolated colonic disease were associated with a high remission rate (Table 2). On multivariate logistic regression analysis, male gender [odds ratio (OR)=1.99, 95% confidence interval (CI): 1.06–3.73, p=0.032] and isolated colonic disease (OR=4.96 vs. small bowel disease, 95% CI: 1.68–14.67, p=0.004) were significantly associated with the remission rate. The other factors were not associated with the remission rate at week 14.
Among 165 patients who had a partial or complete response at week 14, the estimated rates of sustained clinical response were 96.2% at week 30 and 88.0% at week 54, respectively, on Kaplan-Meier analysis. In 111 patients with complete response (remission) at week 14, the estimated rates of sustained clinical remission were 93.3% at week 30 and 77.0% at week 54, respectively (Fig. 3). Log-rank test results showed that no clinical factors were associated with the remission rate at week 30 as well as at week 54, including sex, time from diagnosis to first infusion of infliximab, and concomitant IMM use.
Of 231 patients, 170 received ileocolonoscopy before infliximab therapy. Active ulcerations and a cobblestone appearance were found in 141 (82.9%) and 65 (38.2%) patients, respectively. Among 54 patients who underwent follow-up ileocolonoscopy, mucosal healing was present in 32 (59.3%). The mean interval between the initial and follow-up ileocolonoscopies was 33.7±28.9 (range 2–121 months). There were no clinical factors associated with the mucosal healing rate.
To evaluate the long-term efficacy, we performed a subanalysis in the 185 patients who were followed-up for 6 months or more. The mean follow-up period was 28.3±18.4 months (range 6–103 months). At the time of the last follow-up, the mean CDAI score was 122.3±84.4 (range 10.6–475). Of the 185 patients, 111 (60.0%) were in remission, 54 (29.2%) had mild disease, and 20 (10.8%) had moderate or severe disease based on CDAI scores. The remission rate was higher in patients with mucosal healing than in those without mucosal healing on the follow-up ileocolonoscopies (78.1% vs. 50.0%, p=0.031).
Among the 119 patients who were diagnosed with fistulizing CD or both luminal and fistulizing CD, the efficacy of induction therapy was assessed at week 14 in 73 patients who received three doses of infliximab. Clinical response and remission were observed in 62 (85.0%) and 41 patients (56.2%), respectively (Fig. 4). The clinical response and remission rates tended to be higher in patients with external fistulas than in those with internal fistulas (87.3% and 58.2% for external fistulas vs. 69.2% and 46.2% for internal fistulas, respectively), and among the patients with external fistulas, these rates tended to be higher in patients with perianal fistulas than in those with enterocutaneous fistulas (89.6% and 56.3% for perianal fistulas vs. 77.8% and 66.7% for enterocutaneous fistulas, respectively) without statistical significance. No other clinical factors were associated with the remission rate at week 14 (Table 3).
Among the 62 patients who had a partial or complete response at week 14, three failed to maintain sustained clinical response at weeks 30 and 54. In 41 patients with complete response (remission) at week 14, one did not maintain sustained clinical remission at weeks 30 and 54. The estimated rates of sustained clinical response and remission were 94.0% and 97.1%, respectively, at both weeks 30 and 54. The predictive factors for the sustained remission were not analyzed, as only one patient failed to sustain remission at weeks 30 and 54.
To evaluate the long-term efficacy, we performed a subanalysis in the 104 patients who were followed-up for 6 months or more. The mean follow-up period was 30.3±19.0 months (range 6–103 months). At the time of the last follow-up, the clinical response and remission rates were 78.8% (82 of 104) and 54.8% (57 of 104), respectively.
During the follow-up period, 34 patients (10.7%) had new CD-related complications as follows: 27 strictures, three perianal fistulas, three intra-abdominal abscesses accompanying internal fistulas, and one case of intractable GI bleeding. Twenty-seven patients (8.5%) underwent operations as follows: seven anal fistulectomies (or fistulotomies), four seton operations, 15 segmental bowel resections, and one total proctocolectomy and ileostomy.
Overall, 39 patients (12.3%) experienced an adverse event during infliximab therapy (Table 4). All of the adverse events could be directly attributed to infliximab. Two of the eight patients who had chronic hepatitis B or were hepatitis B virus carriers experienced mild elevation of alanine aminotransferase, although these responses spontaneously improved. Eighteen patients (5.7%) discontinued infliximab due to serious adverse events: seven cases of severe infusion reactions, two cases of serum sickness, two cases of intra-abdominal abscess, and seven cases of active tuberculosis. There were no mortalities in this study.
In terms of tuberculosis, seven cases of active pulmonary TB developed during infliximab therapy. Two of these patients had negative results in both latent TB screening tests (TST and IGRA), and three did not undergo either latent TB test. One patient had a past history of pulmonary TB and scar change on her chest X-ray and received anti-TB prophylaxis for 9 months with isoniazid (INH), with initiation of infliximab after 3 weeks of INH medication. In all seven patients, pulmonary TB was cured with anti-TB medications; however, infliximab treatment was restarted in only one patient who continued infliximab therapy for 28 months without TB reactivation. Detailed information on patients with active TB is provided in Table 5.
This report presents a relatively large-scale Asian study on the efficacy and safety of infliximab therapy for CD. In patients with active luminal CD, clinical response and remission rates were 89.2% and 60.0%, respectively, at week 14. The estimated rates of sustained clinical response and remission were 96.2% and 93.3% at week 30 and 88.0% and 77.0% at week 54, respectively, among the week-14 responders. For fistulizing CD, clinical response and remission rates were 85.0% and 56.2%, respectively, at week 14, and the clinical responses were maintained in most of the week-14 responders at both weeks 30 and 54. The initial responses of infliximab on fistulizing CD tended to be more effective in patients with external fistulas, more so in those with perianal fistulas than in those with internal fistulas.
The results for the response to induction therapy in our study are comparable to those of previous studies that used infliximab, which reported early response rates of 58–96% and remission rates of 40–73%.2122232425 However, the sustained response and remission rates in patients with luminal CD were slightly higher than those of previous randomized, controlled trials (RCTs) such as the ACCENT-I study, in which the clinical response and remission rates were 50% and 39% at week 30 and 39% and 30% at week 54, respectively.18 Additionally, the estimated response rates of maintenance treatments in patients with fistulizing CD in our study were higher than those in the ACCENT-II study in which the proportions of patients with response and remission were 46% and 36% at week 54, respectively.19 This disparity in response rates may be explained by differences in the characteristics of the patients (ethnicity, indications for infliximab, disease severity, and extension) or in the study design (RCT or not).
Current treatment goals for CD are changing from the induction of symptomatic remission to the achievement of mucosal healing, as the latter leads to improved long-term clinical outcomes.2627 Mounting evidence suggests that achieving mucosal healing may change the natural course of the disease by decreasing the need for surgery and reducing hospitalization rates of CD patients.28 Mucosal healing is now recognized as a new therapeutic goal in CD, at least in clinical trials.29 Consistent with this approach, mucosal healing was associated with a higher long-term remission rate in our study. However, this result must be validated in a future prospective study.
In the analysis of the factors related to clinical remission at week 2 for luminal CD, patients whose first infusion of infliximab was less than 3 years from diagnosis had higher remission rates than those whose first infusion occurred more than 3 years after diagnosis. This is compatible with previous reports that have convincingly shown that anti-TNF therapy is more effective in patients with a short disease history.3031 However, the disease duration from diagnosis to first infusion of infliximab was not associated with clinical remission at weeks 6 and 14. In the analysis to identify factors affecting the remission rate at week 14, sex and disease location were significantly associated with the remission rate. However, the disease location was not associated with response rates at weeks 2 and 6. Thus, we cannot confidently suggest the predictive factors for initial response to infliximab due to the inconsistency of the results. A future prospective study may confirm the possibility of sex, disease location, and duration as predictive factors for initial response to infliximab in luminal CD.
No factors were associated with the efficacy of maintenance therapy in luminal CD, including sex, disease location, and duration. The SONIC trial demonstrated that the combination of infliximab and azathioprine (AZA) was superior to either infliximab or AZA alone in patients with active luminal CD naive to IMMs and biologics.32 A large portion of the patients in our study (76.7%) had experienced IMMs before infliximab therapy; therefore, the response rates did not appear to be different between the combination therapy of infliximab plus AZA and infliximab alone. In addition, patients with high baseline C-reactive protein, those with isolated colonic disease and no previous abdominal surgery, young patients, and nonsmokers have all been suggested to be more likely to respond to anti-TNF therapy.9 However, these factors were not related to the maintenance effect of infliximab therapy in our study. Only isolated colonic disease was associated with initial response. These discrepancies might also originate from differences in the characteristics and ethnicity of the study population or the study design (prospective or retrospective). Factors related to the efficacy of maintenance therapy using infliximab also need to be investigated more in a prospective study.
Induction and maintenance therapy with infliximab was well tolerated in Korean patients with CD. Overall, 39 patients (12.3%) experienced an adverse event during infliximab therapy. As this study was conducted retrospectively, the overall incidence of adverse events, including infusion reactions and infections (not serious) might have been lower than in the ACCENT-I and II trials. However, the reported incidence of serious adverse events was similar to those in previous studies.1819 In this study, 19 patients (6.0%) had serious adverse events, including seven cases of active pulmonary tuberculosis. Fortunately, there were no mortalities, and all cases of active TB were safely cured with anti-TB medications.
TB is significantly more common in South Korea than in other developed countries. In the USA, the incidence rate of TB was 3.2 per 100000 person-years in 2012 and is currently in decline, 33 whereas the incidence of TB in the general Korean population is 92 per 100000 person-years.34 Moreover, anti-TNF therapy was shown to be associated with a 2.5- to 18-fold increase in the incidence of TB infection compared to healthy controls, although the actual incidence was not particularly high.343536373839 Thus, screening tests to confirm active TB and LTBI prior to starting anti-TNF therapy are essential. According to the Korean guidelines for TB, patients who are to be treated with anti-TNF agents should first be checked regarding their past TB history, contact history with patients with TB, and present suspected symptoms of TB and should have a chest X-ray examination and TB infection tests.3440 The TB infection tests should be performed by using an IGRA alone or both an IGRA and a TST. A TST alone is not recommended for testing TB infection in patients with inflammatory bowel disease, as the use of immunosuppressive agents may lead to a false negative TST result due to anergy.3440 In our study, the incidence of active TB was relatively high; moreover, two patients had negative results in TB infection tests (TST and IGRA) and normal chest X-ray results, and another patient received anti-TB prophylaxis (INH, 9 months) due to LTBI among the seven patients in whom active TB developed. Thus, a screening test for TB infection using both a TST and an IGRA at one time before starting infliximab may not be sufficient to prevent active TB development, particularly in countries with a high prevalence of TB. Physicians should thoroughly review patient histories, TST and IGRA results, and chest X-ray findings before commencing anti-TNF therapy for CD, and they should also pay attention to the risk of TB development even if the patients do not have LTBI or have taken treatment for LTBI. In addition, a substantial portion of TB cases (71%, 5 of 7) occurred within 3 months after infliximab initiation. Thus, more attention to TB development may be needed during the early period of infliximab therapy.
A limitation of our study was its retrospective design. Moreover, we did not evaluate trough levels and antibodies to infliximab. Nonetheless, this was a relatively large-scale, nationwide, multicenter study to evaluate the efficacy and safety in Korean patients with CD, and it provided baseline data for subsequent well-designed prospective studies.
In conclusion, we have demonstrated the efficacy of infliximab in induction and maintenance treatments in Korean patients with luminal and fistulizing CD. Infliximab treatment was safe and well tolerated. However, physicians should always be cautious about the possible adverse effects of infliximab, particularly for TB. Further prospective studies are needed to evaluate the long-term efficacy and safety of infliximab therapy and to determine the predictors of response in patients with CD.
Figures and Tables
Fig. 2
Clinical response (complete and partial response) and remission (complete response) at weeks 2, 6, and 14 in patients with active luminal Crohn's disease.
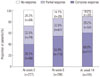
Fig. 3
(A) Cumulative probabilities of sustained clinical response for week 14 responders and (B) cumulative probabilities of sustained clinical remission for patients in remission at week 14 among patients with active luminal Crohn's disease.

Fig. 4
Clinical response (complete and partial response) and remission (complete response) at week 14 in patients with fistulizing Crohn's disease.
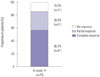
Table 1
Baseline Characteristics of the Patients

Table 2
Predictive Factors Related to Clinical Remission at Week 14 in Luminal Crohn's Disease

Table 3
Predictive Factors Related to Clinical Remission at Week 14 in Fistulizing Crohn's Disease

Table 4
Adverse Events during Infliximab Therapy
Table 5
Details of Tuberculosis Infection

References
1. Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104:465–483.

2. Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis. 2010; 4:7–27.

3. Tozer PJ, Whelan K, Phillips RK, Hart AL. Etiology of perianal Crohn's disease: role of genetic, microbiological, and immunological factors. Inflamm Bowel Dis. 2009; 15:1591–1598.

4. Ford AC, Kane SV, Khan KJ, Achkar JP, Talley NJ, Marshall JK, et al. Efficacy of 5-aminosalicylates in Crohn's disease: systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:617–629.

5. Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:630–642.

6. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007; 5:103–110.

7. Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993; 30:1443–1453.

8. Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997; 159:6276–6282.
9. D'Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011; 106:199–212.
10. Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4:28–62.

11. Hamzaoglu H, Cooper J, Alsahli M, Falchuk KR, Peppercorn MA, Farrell RJ. Safety of infliximab in Crohn's disease: a large single-center experience. Inflamm Bowel Dis. 2010; 16:2109–2116.
12. Park SK, Ye BD, Lee C, Im JP, Kim YH, Kim SO, et al. Risk and clinical characteristics of lymphoma in Korean patients with inflammatory bowel diseases: a multicenter study. J Clin Gastroenterol. 2015; 49:e11–e16.
13. Ng SC, Tsoi KK, Kamm MA, Xia B, Wu J, Chan FK, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012; 18:1164–1176.

14. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012; 10:1002–1007.

15. Jo KW, Hong Y, Park JS, Bae IG, Eom JS, Lee SR, et al. Prevalence of latent tuberculosis infection among health care workers in South Korea: a multicenter study. Tuberc Respir Dis (Seoul). 2013; 75:18–24.

16. Lee CH, Jeong YJ, Heo EY, Park JS, Lee JS, Lee BJ, et al. Active pulmonary tuberculosis and latent tuberculosis infection among homeless people in Seoul, South Korea: a cross-sectional study. BMC Public Health. 2013; 13:720.

17. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6.

18. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.

19. Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004; 350:876–885.

20. Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004; 50:1051–1065.

21. Ardizzone S, Colombo E, Maconi G, Bollani S, Manzionna G, Petrone MC, et al. Infliximab in treatment of Crohn's disease: the Milan experience. Dig Liver Dis. 2002; 34:411–418.

22. Kim YJ, Kim JW, Lee CK, Park HJ, Shim JJ, Jang JY, et al. Clinical outcome of treatment with infliximab in Crohn's disease: a single-center experience. Korean J Gastroenterol. 2013; 61:270–278.

23. Orlando A, Colombo E, Kohn A, Biancone L, Rizzello F, Viscido A, et al. Infliximab in the treatment of Crohn's disease: predictors of response in an Italian multicentric open study. Dig Liver Dis. 2005; 37:577–583.

24. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999; 340:1398–1405.

25. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease Crohn's Disease cA2 Study Group. N Engl J Med. 1997; 337:1029–1035.

26. Bouguen G, Levesque BG, Feagan BG, Kavanaugh A, Peyrin-Biroulet L, Colombel JF, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13:1042–1050.e2.

27. D'Haens GR, Sartor RB, Silverberg MS, Petersson J, Rutgeerts P. Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014; 8:726–734.
28. Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Results from the 2nd Scientific Workshop of the ECCO I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis. 2011; 5:477–483.

29. Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111.e2.

30. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.

31. D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008; 371:660–667.
32. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.

33. Centers for Disease Control and Prevention (CDC). Trends in tuberculosis--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62:201–205.
34. Shim TS. Diagnosis and treatment of latent tuberculosis infection in patients with inflammatory bowel diseases due to initiation of anti-tumor necrosis factor therapy. Intest Res. 2014; 12:12–19.

35. Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010; 69:522–528.

36. Lee SK, Kim SY, Kim EY, Jung JY, Park MS, Kim YS, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013; 191:565–571.

37. Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderateto-severe Crohn's disease. Am J Gastroenterol. 2009; 104:2524–2533.

38. Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Bréban M, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009; 60:1884–1894.

39. Yoo IK, Choung RS, Hyun JJ, Kim SY, Jung SW, Koo JS, et al. Incidences of serious infections and tuberculosis among patients receiving anti-tumor necrosis factor-α therapy. Yonsei Med J. 2014; 55:442–448.

40. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Cheongju: Korea Centers for Disease Control and Prevention;2014.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download