Abstract
We report herein a case of benign cardiac schwannoma in the interatrial septum. A 42-year-old woman was transferred from a clinic because of cardiomegaly as determined by chest X-ray. A transthoracic echocardiography and chest computed tomography examination revealed a huge mass in the pericardium compressing the right atrium, superior vena cava (SVC), left atrium, and superior pulmonary vein. To confirm that the tumor originated from either heart or mediastinum, cine magnetic resonance imaging was performed, but the result was not conclusive. To facilitate surgical planning, we used 3D printing. Using a printed heart model, we decided that tumor resection under cardiopulmonary bypass (CPB) through sternotomy would be technically feasible. At surgery, a huge tumor in the interatrial septum was confirmed. By incision on the atrial roof between the aorta and SVC, tumor enucleation was performed successfully under CPB. Pathology revealed benign schwannoma. The patient was discharged without complication. 3D printing of the heart and tumor was found to be helpful when deciding optimal surgical approach.
A 42-year-old woman was transferred from a clinic because of the incidental detection of cardiomegaly by chest X-ray (Fig. 1A) during a health examination. She had felt palpitations on several occasions, but had not sought medical advice. Her initial electrocardiogram (EKG) showed ectopic atrial rhythm with right axis deviation. Transthoracic echocardiography revealed a huge mass compressing the right atrium (RA). Chest computed tomography (CT) revealed a 10×9.5 cm sized heterogeneously enhanced mass compressing the superior vena cava (SVC), RA, left atrium (LA), and superior pulmonary vein (PV) (Fig. 1B). Under the impression of a malignant mediastinal tumor, the patient was transferred to our cardiothoracic surgery department. After reviewing her chest CT, we thought that the base of mass was at the interatrial septum rather than at an extracardiac position. To confirm the mass origin, we performed cine magnetic resonance imaging (MRI), but the origin of the mass remained equivocal (Fig. 1C). MRI showed a large mass with central necrosis in the right paracardiac area extending to the right side of the ascending aorta between the SVC and right superior PV. Enhancing solid portion of the mass at the periphery shows intermediate to high signal intensity on T1WI and T2WI. These findings most likely represent a primary pericardial tumor such as sarcoma, malignant mesothelioma, germ cell tumor, and hemangioma. To facilitate surgical planning, we used 3D printing. After converting the CT image into a 3D image using Mimics Base version 16 (Materialize, Leuven, Belgium), the segmented 3D image was stored as a stereolithography (STL) file (Fig. 2A). The file was then sent to a 3D printer (uPrint, Stratasys Ltd., MN, USA), and a 3D model of the heart was printed (Fig. 2B). Based on an examination of the printed heart model, we decided that tumor resection under cardiopulmonary bypass (CPB) through sternotomy would be technically feasible. At surgery, a huge oval mass with a broad base which was located at the atrial roof between the aorta and the SVC was confirmed (Fig. 2C). For CPB, the aorta, SVC, and right femoral vein were cannulated. By incision on the atrial roof between the aorta and the SVC, tumor enucleation was performed under CPB. The tumor did not infiltrate into RA or LA, and thus, interatrial septal wall repair was unnecessary (Fig. 2D). The CPB time was 170 minutes and aortic cross clamp time was 93 minutes. Grossly, the mass was 14×10×7 cm sized, weighed 370 g, and was lobulated, encapsulated, yellowish, and contained a hemorrhagic region (Fig. 3A). Microscopic findings revealed the presence of Antoni A and B bodies (Fig. 3B), and positive immunohistochemistry for S-100 protein. These findings were compatible with benign schwannoma. The postoperative coronary artery CT showed clear resection margin (Fig. 3C). The patient was discharged without complication such as symptoms related with the vagus nerve or cardiac plexus.
Primary cardiac schwannoma is extremely rare; only 17 cases have been reported in the literature.1 Primary schwannoma is believed to originate from the cardiac plexus or the cardiac branch of the vagus nerve, and thus, most reported cardiac schwannomas have involved the right side heart,12 but left-side schwannomas have also been reported.3 In the present case, the base of mass was in the interatrial septum, but it did not infiltrate into the right or left atrial cavity. We believe that this is the first case of schwannoma in the interatrial septum removed by enucleation alone. Because the tumor had a broad base in the interatrial septum and there was no tumor stalk, it was difficult to confirm preoperatively that the tumor had a cardiac rather than a mediastinal originated origin. This meant that surgical planning was problematic. However, after examining the 3D printed model, we decided that sternotomy approach and tumor removal under CPB was the more appropriate option.
Several groups have tried to use 3D heart models for planning heart surgery.4 These models simplify surgical procedures, such as resections of ventricular aneurysms or malignant cardiac tumors, because they improve preoperative planning and knowledge of the intraoperative dispositions of structures at risk and target tissues, and thus, might improve surgical outcomes.4 In our case, 3D printing was found to be helpful when deciding the approach used, although the CT data set used was obtained using a slice thickness of 5.0 mm, and thus, segmentation result was rather coarse as was the margin between the heart and tumor. Accordingly, we believe that the use of data sets obtained using thinner slices would be even more helpful during surgical planning.
Because CT or MRI image is used for 3D printing model, it is not easy to get more exact anatomical information such as the tumor infiltration, or tumor stalk which is not conclusively shown on the CT or MRI by 3D printing model. In the present case, we could not get the information about exact origin of tumor by the 3D printing, nevertheless, the model was helpful for clearly identifying the tumor position and relationship with the great vessels. It is unarguable that CT or MRI is very useful for diagnosis cardiovascular disease because these images provides high-quality data, however, in some cases, these images may not be enough for ideal perioperative planning and decision-making due to complex cardiovascular pathologies.5 Because the true-to-life 3D models demonstrate anatomical structures and provide haptic perception, 3D printing models are valuable tools for perioperative planning and might be more excellent than other imaging techniques in some cases.5
Figures and Tables
Fig. 1
Chest X-ray and computed tomograph obtained before stent grafting. (A) Chest X-ray showing an extended right heart silhouette. (B) CT revealing a 10×9.5 cm sized heterogeneously enhanced mass in the right pericardial space compressing the superior vena cava, right atrium, left atrium, and superior pulmonary vein. (C) MRI showing a large mass with central necrosis in the right paracardiac area extending to the right side of the ascending aorta between the SVC and right superior PV. SVC, superior vena cava; PV, pulmonary vein.

Fig. 2
3D printing model and operating finding. (A) The segmented 3D image using Mimics Base version 16 (Materialize, Leuven, Belgium) showed a huge cardiac mass (arrow). (B) The model of heart and mass which was printed by 3D printer (uPrint, Stratasys Ltd., MN, USA) showed a huge mass (arrow). (C) After sternotomy, a huge yellowish mass was confirmed at Waterstone's groove (arrow). (D) After tumor removal, repair of the interatrial septum was unnecessary, because the tumor did not infiltrate the right or left atrial cavities. Arrow indicates the position where the tumor occupied. SVC, superior vena cava; RA, right atrium.
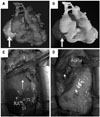
Fig. 3
Pathologic findings and postoperative CT. (A) Macroscopically, the mass was capsulated, nobular, and yellowish, and contained hemorrhagic part. (B) The microscopic finding showed Antoni A area, with hypercellularity composed of palisading nuclei and fibers, and Antoni B area with hypocellularity. (C) Postoperative coronary artery CT showed that there was no remnant tumor.

ACKNOWLEDGEMENTS
This work was supported by the Gachon University Gil Medical Center (Grant number: FRD2013-15).
References
2. Nakamura K, Onitsuka T, Yano M, Yano Y. Surgical resection of right atrial neurilemoma extending to pulmonary vein. Eur J Cardiothorac Surg. 2003; 24:840–842.

3. Monroe B, Federman M, Balogh K. Cardiac neurilemoma. Report of a case with electron microscopic examination. Arch Pathol Lab Med. 1984; 108:300–304.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download