Abstract
Purpose
The adult tetanus, reduced diphtheria, and acellular pertussis (Tdap) vaccine has been introduced in order to provide individual protection and reduce the risk of transmitting pertussis to infants. We assessed the knowledge and acceptability of the Tdap vaccine around pregnancy.
Materials and Methods
This study was a cross-sectional survey of women of childbearing age (20-45 years) who visited obstetrics and gynecologic units of primary, secondary, or tertiary hospitals. They were asked to fill in a questionnaire assessing their knowledge, attitudes, and acceptability of Tdap.
Results
The questionnaire was completed by 308 women; 293 (95.1%) had not received information from doctors about Tdap, and 250 (81.2%) did not know about the need for vaccination. A significantly important factor related to subjects' intention to be vaccinated, identified by stepwise multiple logistic regression, was the knowledge (OR 13.5, CI 3.92-46.33) that adult Tdap is effective in preventing pertussis for infants aged 0-6 months. Additionally, 276 (89.6%) considered the recommendation of obstetric doctors as the most influencing factor about Tdap vaccination.
Conclusion
In Korea, most women of childbearing age seem to be neither recommended nor adequately informed about the vaccination, although our population was not a nationwide representative sample. Information given by healthcare workers may be critical for improving awareness and preventing pertussis.
Pertussis, or whooping cough, is a highly contagious respiratory tract disease caused by Bordetella pertussis. Pertussis can be an important cause of morbidity and mortality in young infants under the age of 6 months, although it affects people of all ages.1 Despite widespread immunization against pertussis among infants and children since the 1950s, pertussis has remained endemic. There has been gradual increase after a nadir in 1976, and substantial increases since 2000.2 The waning of vaccine-acquired immunity and decreased opportunities for boosting of immunity are considered as main reasons for the reemergence of pertussis.2,3,4
Perinatal vaccination of close contacts of infants is known as cocooning. Since 2006, the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) has recommended tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination for household contacts of very young infants, with particular emphasis on mothers getting vaccinated in the postpartum period.5
Korean government began to recommend routine diphtheria, tetanus, and whole cell pertussis (DTwP) vaccination in 1954 and introduced the diphtheria, tetanus, and acellular pertussis (DTaP) vaccine into the National Immunization Program in 1989. It has since been successful in controlling pertussis, with low numbers of reported cases until the 2000s.6,7 However, there has been increasing in the reported number of pertussis-case patients during the last decade.8 In 2009, the Tdap vaccine was licensed in Korea for use in adolescents (aged more than 11 years) and adults.9
Adults and adolescents in close contact with infants are a potential transmission source of pertussis.10 Therefore, awareness and understanding in women of childbearing age is critical for effective Tdap vaccine implementation. This study aimed to assess the knowledge of pertussis infection among women of childbearing age and their attitudes towards vaccination with the Tdap vaccine.
This study was a cross-sectional survey of women of childbearing age (20-45 years) living in Seoul and the surrounding area (25000000 inhabitants). The survey was a self-administered close-ended questionnaire and it was conducted with the other survey, in a same way.11 Participants were asked to fill in the questionnaire in the clinic in order to assess their knowledge, attitudes, and acceptability of Tdap vaccination. Among women of childbearing age who visited the hospitals, those who were planning a pregnancy, pregnant, or within 6 months postpartum were included in this study. Both the study and waiver of written consent were approved by the respective Institutional Review Boards.
The questionnaire used in this study was adapted from a previous questionnaire that had been established and validated by a Taiwanese group.12 The three-page questionnaire of 18 multiple choice questions included respondents' demographic characteristics, perceived risk of infant pertussis, concern about the safety of Tdap, access to information about Tdap, possible barriers about Tdap, and, finally, the most important factor influencing a woman's decision to accept or decline the vaccine.
The primary outcome variable was each woman's response to the question about their intention to accept or decline Tdap given the assumption that maternal Tdap effectively prevents infant pertussis. We analyzed the frequency of each response in relation to all variables including demographic characteristics, knowledge, attitude, and risk perception in this study. A chi-square test or Fisher's exact test was used to identify significant associations between women's intentions to vaccinate or not and their survey responses. All variables with p<0.10 in univariate analyses were entered into the multivariable logistic model to identify factors associated with acceptance or declination of Tdap immunization. Variables that had originally been entered into the model were then removed if considered non-significant, and this was done in a stepwise fashion. All statistical analyses were conducted using SAS version 12.1. A p-value of 0.05 or less was considered significant.
During the study period, 437 women of childbearing age residing within the geographic area were approached, and of these, 342 were planning pregnancy, pregnant, or within 6 months postpartum and therefore eligible to participate. Ultimately, 317 of the eligible women agreed to participate, and 308 had complete survey responses, resulting in a complete survey response rate of 97.2%. Demographic characteristics are presented in Table 1.
Baseline characteristics of total population was same with the previous publication.11 Groups were separated according to intention regarding Tdap vaccination. There was no significant difference among groups according to maternal age, parity, education level, pregnancy status, monthly family income level, or occupation.
Women's knowledge about pertussis and attitudes in relation to Tdap vaccination before, during, and after pregnancy are presented in Table 2. Significant differences were found between women who intended to accept Tdap and those who did not intend to accept Tdap in 1) the rated level of contagiousness of pertussis (p=0.026) and 2) the perceived effectiveness of Tdap in preventing infant pertussis (p<0.001). Most women (95.1%) replied that they had not received information from doctors about Tdap vaccination. Only 3.9% of women replied that they had either received Tdap vaccination or did not know whether they had received it. The most common reason why they had not received Tdap vaccination before, during, or after pregnancy was that they had not known of the need for Tdap vaccination (81.2%).
In comparing women who intended to accept or decline Tdap vaccination, only one variable became significant in the final logistic regression model. Women who intended to accept Tdap vaccination were more likely to think that adult pertussis vaccination is highly effective in preventing pertussis for infants aged 0-6 months, with an odds ratios of 13.5 (95% confidence interval, 3.92-46.33, p=0.002) (Table 3).
From a list of 12 factors, respondents selected the single most important factor that influenced their intention to accept or decline Tdap. The three most frequently selected reasons for respondents' intention of accepting Tdap were belief that the respondent would be the most likely source of pertussis infection for her baby (72.4%), belief that the vaccine might be helpful for her baby's health (15.2%), and believing pertussis is a severe disease among newborn infants (7.1%) (Table 4).
On the other hand, the three most frequently selected reasons for respondents' intention of declining Tdap were 1) concern about the side effects of Tdap (64.0%), 2) believing the respondent's baby would not be at high risk for developing pertussis (16.0%), and 3) not receiving a recommendation by medical doctors (12.0%). Most women (90.6%) did not know that the Korean government recommends an adult pertussis vaccination before pregnancy, during pregnancy (in case of outbreak), or during the postpartum period (Table 5). Regarding possible information or recommendation sources, 276 women (89.6%) replied that the most influencing factor in vaccination would likely be recommendation by medical staff in the obstetrics and gynecology department.
Unsurprisingly, only 3.9% of women replied that they had either received Tdap vaccination or did not know whether they received it. The most common reason why they had not received Tdap vaccination before, during, or after pregnancy was that they had not known the need for Tdap vaccination. Most women replied that they had not received information from doctors about Tdap. Women who had no intention of receiving Tdap were more likely to rate maternal or infant risk of exposure to pertussis as low and to report that they did not know the efficacy of Tdap vaccination. These responses were similar to those of Taiwanese women who declined postpartum Tdap.12 Even in Canada, baseline knowledge of infant disease severity and adult vaccine recommendations was still poor, although the government there has recommended a single Tdap booster for all adults from 2008, especially encouraging parents so as to create a cocooning effect for infants.13 In Australia, a study reported that 75% of mothers and 69% of fathers were aware that the pertussis vaccine was available and funded for new parents in a state that has funded Tdap for parents since 2009.14 Government support seems essential not only to increase vaccine uptake but also to increase awareness.
The trend of increasing pertussis cases is now becoming obvious in Korea, although it is less common than in Western countries. The reported number of pertussis cases increased up to 66 cases in 2009.8 Considering that the diagnosis of reported cases was confirmed by polymerase chain reaction and culture without serology, the actual number of cases was likely much more than the reported number. Since 2011, the US ACIP has expanded its recommendation to include Tdap vaccination during the late-second or third trimester of pregnancy, as well as in the postpartum period, irrespective of the patient's prior history of receiving Tdap, as the incidence of pertussis cases was not decreasing and uptake of Tdap was very low.15 The UK authorities did the same recommendation in October 2012, when they faced a rapid rise in pertussis cases and associated infant deaths that year.16
In Korea, it has been reported that approximately 20% of health care workers are susceptible to pertussis and that the immunity to pertussis gradually decreases after 15 years of age.17,18 In Korean pediatric hematology and oncology patients, serologic immunity to Diphtheria-Tetanus-Pertussis was found to be much lower than immunity in a healthy pediatric population.19 It has also been reported that household members, mainly parents, were responsible for pertussis transmission in the serologically-confirmed pertussis cases in Korean infants younger than 6 months of age.20 Ideally, a nationwide epidemiologic study of pertussis based on serology should be undertaken to develop diagnosis criteria for atypical pertussis and to set up vaccination strategies that can prevent waning immunity and reduce the number of very young unvaccinated individuals. Currently however, control of pertussis through booster vaccination with Tdap in families taking care of young infants is of immediate necessity in Korea. Therefore, the Committee on Infectious Disease in the Korean Pediatric Society and Korean CDC recommended that mothers of newborn infants should be given a dose of Tdap as soon as is feasible if they previously have not received Tdap.9,21 Other candidates for Tdap include health care workers and family members who contact infants younger than 12 months old, as well as childbearing-aged women, baby-care center workers, etc. Although pregnancy is not a contraindication to Tdap immunization, it is only recommended in Korea during pregnancy if clinically indicated, such as during an outbreak.21
Our study results demonstrated that women of childbearing age had insufficient knowledge about pertussis and related immunization recommendations and were consequently under-immunized. National pertussis surveillance data in Korea demonstrated that the most common age groups affected during 2010-2011 were those aged <3 months and ≥15 years.22 Because infants without timely vaccination are at increased risk of pertussis infection, steps to provide timely vaccination to infants and to provide Tdap vaccination to adolescents and adults is now being tried in Korea. Moreover, enhanced surveillance to discover adult pertussis-case patients with atypical symptoms should be proceeded in order to prevent transmission to vulnerable infants. Financial support and adequate information for Tdap vaccination from the government may lead to a decrease of cases in the susceptible population among adolescents and adults.
Despite national recommendations in several countries, uptake in pregnant women, mothers after delivery, and household contacts of infants remains limited. It seems that multiple strategies to increasing vaccine uptake rate should be considered to increase protection in infants, such as, not only dissemination of information and education, but also consideration of easy accessibility and best timing for decision making, as well as increase of trust.12,23 The postpartum period seems an opportune and unique time to provide pertussis vaccination to previously unimmunized mothers. In a recent study, when a Tdap vaccine was offered in the community after the promotion of vaccination in the maternity ward, the uptake rate did not significantly improve.14,24 When promotion and administration of the vaccine in the maternity ward was provided, the uptake rate increased up to 50% of parents, approximately.13,14 Therefore, offering Tdap in the maternity ward may be an effective approach for promoting cocooning and increasing vaccine uptake. A recent randomized trial reported that there was no increased risk of adverse events among women who received Tdap vaccine during pregnancy or their infants, and maternal immunization with Tdap resulted in high concentrations of pertussis antibodies in infants during the first 2 months of life without substantially altering infant responses to DTaP.25 Vaccination strategy during pregnancy could be also considered as a means of increasing vaccine uptake.
As our study demonstrated that recommendation from medical staff in obstetrics and gynecology departments is the most influencing factor in vaccination, recommendations for Tdap provided with appropriate information by Korean maternal-fetal medicine professionals may be critical for improving awareness. Additionally, vaccination in the maternity ward after vaginal or cesarean delivery may also prove beneficial for preventing pertussis, although our population is not a nationwide representative sample of women of childbearing age. Further investigation of the effectiveness of the cocooning strategy in decreasing infant morbidity and mortality is required in Korea, as is a nationwide epidemiologic study of pertussis.
Figures and Tables
Table 1
Characteristics According to Intention of Tdap Vaccination
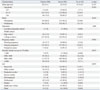
Table 2
Knowledge and Attitudes Related to Tdap According to Intention of Pertussis Vaccination

Table 3
Factors Associated with Intention of Tdap Vaccination

Table 4
The Single Most Important Factor That Influences One's Intention to Accept or Decline Tdap
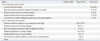
Table 5
Information or Recommendation Sources That Can Affect Tdap Vaccination
References
1. Wood N, Quinn HE, McIntyre P, Elliott E. Pertussis in infants: preventing deaths and hospitalisations in the very young. J Paediatr Child Health. 2008; 44:161–165.

2. Lin YC, Yao SM, Yan JJ, Chen YY, Chiang CS, Wu HS, et al. Epidemiological shift in the prevalence of pertussis in Taiwan: implications for pertussis vaccination. J Med Microbiol. 2007; 56(Pt 4):533–537.

3. Sin MA, Zenke R, Rönckendorf R, Littmann M, Jorgensen P, Hellenbrand W. Pertussis outbreak in primary and secondary schools in Ludwigslust, Germany demonstrating the role of waning immunity. Pediatr Infect Dis J. 2009; 28:242–244.

4. Duranoglu L, Sönmez C, Vurucu S, Kurtoglu D, Kesik V, Coplu N, et al. Evaluation of pertussis immunity status in schoolchildren immunized with whole-cell vaccine. Epidemiol Infect. 2010; 138:299–303.

5. Murphy TV, Slade BA, Broder KR, Kretsinger K, Tiwari T, Joyce PM, et al. Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008; 57(RR-4):1–51.
7. Chun BC. Public Policy and laws on infectious disease control in Korea: past, present and prospective. Infect Chemother. 2011; 43:474–484.

8. Division of Tuberculosis and Bacterial Respiratory Infections: Korea National Health Institute. incidence of pertussis in Korea. Seoul: Korea National Health Institute;2009. p. 709.
9. Choi KM, Kim KH, Kim YJ, Kim JH, Park SE, Lee HJ, et al. Recommendation for the use of newly introduced Tdap vaccine in Korea. Korean J Pediatr. 2011; 54:141–145.

10. Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007; 26:293–299.

11. Ko HS, Jo YS, Kim YH, Park YG, Moon HB, Lee Y, et al. Knowledge, attitudes, and acceptability about influenza vaccination in Korean women of childbearing age. Obstet Gynecol Sci. 2015; 58:81–89.

12. Cheng PJ, Huang SY, Shaw SW, Kao CC, Chueh HY, Chang SD, et al. Factors influencing women's decisions regarding pertussis vaccine: a decision-making study in the Postpartum Pertussis Immunization Program of a teaching hospital in Taiwan. Vaccine. 2010; 28:5641–5647.

13. Frère J, De Wals P, Ovetchkine P, Coïc L, Audibert F, Tapiero B. Evaluation of several approaches to immunize parents of neonates against B. pertussis. Vaccine. 2013; 31:6087–6091.

14. Donnan EJ, Fielding JE, Rowe SL, Franklin LJ, Vally H. A cross sectional survey of attitudes, awareness and uptake of the parental pertussis booster vaccine as part of a cocooning strategy, Victoria, Australia. BMC Public Health. 2013; 13:676.

15. Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women-- Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013; 62:131–135.
16. Joint Committee on Vaccination and Immunisation (JCVI). JCVI meeting on pertussis immunisation: August 2012. 2012. accessed on 2013 December 28. Available at: http://transparency.dh.gov.uk/2012/09/28/jcvi-pertussis/.
17. Kim S, Oh H, Ham O, Chung MH, Seo W. Susceptibility and factors of pertussis vaccination adherence in Korean health care workers. Am J Health Behav. 2010; 34:45–53.

18. Kang JH. The assessment of DTaP vaccine; age related seroepidemiology of diphtheria, tetanus and pertussis in Korea. The annual report of KFDA. Cheongju, Korea: Food & Drug Administration;2008. p. 783–784.
19. Kwon HJ, Lee JW, Chung NG, Cho B, Kim HK, Kang JH. Assessment of serologic immunity to diphtheria-tetanus-pertussis after treatment of Korean pediatric hematology and oncology patients. J Korean Med Sci. 2012; 27:78–83.

20. Kwon HJ, Yum SK, Choi UY, Lee SY, Kim JH, Kang JH. Infant pertussis and household transmission in Korea. J Korean Med Sci. 2012; 27:1547–1551.

21. Korean Centers for Disease Control and Prevention (CDC). Guidelines of Vaccination for Adult. November 2012. accessed on 2013 December 28. Available at: http://www.cdc.go.kr.
22. Choe YJ, Park YJ, Jung C, Bae GR, Lee DH. National pertussis surveillance in South Korea 1955-2011: epidemiological and clinical trends. Int J Infect Dis. 2012; 16:e850–e854.

23. Iroh Tam PY, Visintainer P, Fisher D. Response to an education program for parents about adult pertussis vaccination. Infect Control Hosp Epidemiol. 2009; 30:589–592.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download