Abstract
Purpose
We evaluated the validity of robotic surgery (RS) for pediatric choledochal cyst (CC) in comparison to open surgery (OS).
Materials and Methods
From January 2009 to April 2013, clinical data from 79 consecutive pediatric patients with CC, who underwent RS (n=36) or OS (n=43) performed by a single pediatric surgeon, were analyzed retrospectively.
Results
In the RS group, the age of the patients was significantly older, compared to the OS group. Operation and anesthesia times were significantly longer in the RS group than the OS group. Fluid input rates to maintain the same urine output were significantly smaller in the RS group than the OS group. The American Society of Anesthesiologists (ASA) physical status, length of postoperative hospital stay, and the incidence of surgical complications did not differ significantly between the two groups.
Conclusion
Although early complications could not be avoided during the development of robotic surgical techniques, RS for pediatric CC showed results comparable to those for OS. We believe that RS may be a valid and alternative surgery for pediatric CC. After further development of robotic surgical systems and advancement of surgical techniques therewith, future prospective studies may reveal more positive results.
Choledochal cyst (CC) is a congenital condition that develops when cystic dilation occurs in all or a portion of the bile ducts. Although CC is an unusual disease in Western countries, it has a relatively high prevalence in East Asian countries, such as Korea, China, and Japan.1 Usually, it is diagnosed in the first few years of life, although it can be discovered early during the antenatal period using prenatal sonography or late during childhood or early adulthood. Approximately two-thirds of patients with this condition are found at a pediatric age. CC has a high likelihood of progressing to hepatobiliary malignancies, and therefore, prompt diagnosis and treatment are essential.2
Several advances in the surgical management of CC have been made, and the current optimal treatment is considered to involve complete excision of the cyst with reliable biliary reconstruction.3 Generally, open surgery (OS) for CC requires a wide incision and leaves a visible abdominal scar, which may be a concern, particularly in women: CC is reportedly more common in women than in men (female:male 4:1 to 3:1).3 Recently, a few articles regarding robotic surgery (RS) for CC have been published.4,5,6 However, there still is a lack of data comparing OS with RS for this condition with which to justify performing RS for the pediatric CC. Therefore, we aimed to evaluate the perioperative outcomes of RS in pediatric CC in comparison to those for OS.
The study was approved by the Institutional Review Board of Yonsei University Medical Center (4-2013-0596). We reviewed the perioperative medical records from 79 consecutive pediatric CC patients who underwent RS (n=36) using the da Vinci™ Robot System (Intuitive Surgical, Inc., Mountain View, CA, USA) or OS (n=43) performed by one pediatric surgeon from January 2009 to April 2013 at Yonsei University Medical Center in Korea. During this period, all consecutive patients were included, except for patients who received another concurrent surgical procedure. Parents or guardians were permitted to make the final decision regarding the operative method to be used on their children, after they received detailed information on RS and OS for CC, including previous surgical data, and an explanation of the advantages and disadvantages of each approach.
Although the data were collected retrospectively from electronic medical records, all variables were prospectively evaluated. Preoperative variables included the demographics of the patient, such as age, sex, weight, height, and body mass index (BMI). Intraoperative variables included operation time, anesthesia time, intraoperative fluid management, urine output, blood loss, and erythrocyte transfusion. The operation time was defined as the duration between the first skin incision and the end of the operation. Anesthesia time was defined as the duration from the moment the patient entered the operating room until the patient was transferred from the post-anesthesia care unit.
The postoperative data included time to taking water, time to starting a liquid diet, length of postoperative hospital stay, and postoperative complications. Postoperative hospital stay was defined as the duration between the day of the operation and the patient's hospital discharge. We recorded complications into two categories: immediate complications were defined as complications that developed during hospital stay; 30-day complications were defined as complications within 30 days after discharge.7,8
Postoperative pain was measured using the Faces, Legs, Activity, Cry, and Consolability (FLACC) Behavior Pain Assessment Tool designed for infants and a verbal numerical rating scale for pain (vNRS, 0=no pain and 10=worst pain imaginable) for children every 6 hours for 36 hours post-operatively by randomized nurses from the admission unit.9
After endotracheal intubation under general anesthesia, an arterial catheter and a peripheral intravenous catheter were additionally established. A right extended subcostal incision was made with or without operative cholangiography. Complete excision of the CC with the creation of a retrocolic Roux-en-Y hepaticojejunostomy was performed in the usual manner.
After endotracheal intubation, an arterial catheter and a central venous catheter were inserted. A supraumbilical semicircular incision was made for insertion of a 12-mm camera port, and carbon dioxide pneumoperitoneum was established with a pressure of <12 mm Hg. Thereafter, 8-mm robotic working ports were placed in three different areas, including the left upper quadrant, right upper quadrant, and right lower quadrant. Additionally, in the left lower quadrant, a 12-mm laparoscopic assist port was positioned. The RS procedures for pediatric CC consist of two stages (pre-docking and docking stage). In the pre-docking stage, after identifying the jejunum by the laparoscopic method, extracorporeal jejunojejunostomy was performed and a remodeled jejunum was placed back into the peritoneal space. After the robotic surgical system was docked, the CC was completely excised and intracorporeal Roux-en-Y hepaticojejunostomy was performed. The RS procedures for pediatric CC have been described in detail in a previous report.6
Under evidence of return of bowl motility, an oral diet was started on the third day after operation in both groups. If the patient did not have evidence of bowel motility until the third day, the oral diet was usually delayed. Water was given first, followed by a liquid and a soft diet. Patient discharge was considered after all diets could be consumed without any discomfort, abdominal pain, or other complications.
Statistical analysis was performed by using IBM SPSS Statistics software, version 19.0 (SPSS Inc., Chicago, IL, USA) and R version 3.0.1. Patient characteristics (age, weight, height, and BMI), intraoperative patient data, length of postoperative hospital stay, and postoperative duration in the intensive care unit were analyzed using Student's t-test. Inter-group differences in the time to taking sips of water, time to starting a clear liquid diet, and complications were compared by using the Mann-Whitney U test. Postoperative pain scores were presented using box plots. Continuous variables are presented as a mean±SD or median (range), and categorical variables are expressed as numbers (percentages). Statistical significance was defined as a p value of <0.05.
Data from 79 consecutive patients who underwent RS (n=36) or OS (n=43) from January 2009 to April 2013 were obtained from elective medical records. One patient in the OS group who underwent combined urology surgery was excluded. Finally, 36 patients who underwent RS and 42 patients who underwent OS were examined, and their data were analyzed.
Patient characteristics are presented in Table 1. The age of the patients at the time of operation was significantly older in the RS group, compared to the OS group (p<0.05). Also, the weight and height of patients were greater in the RS group than those in the OS group (p<0.05). No significant difference in BMI and American Society of Anesthesiologists (ASA) physical status was noted between two groups.
Intraoperative data are summarized in Table 2. The operation and anesthesia times were significantly longer in the RS group than the OS group (p<0.001). The mean total operating time and the mean console time in the RS group were 520 (range 400-865) and 300 (range 185-475) minutes, respectively. Total amounts of intraoperative fluid input were significantly larger in the RS group than the OS group. However, urine output rates were similar for both groups (0.03±0.02 mL-1kg-1hour in RS group, 0.03±0.03 mL-1kg-1 hour in OS group, p=0.177), while fluid input rates were significantly less in the RS group than the OS group (0.17±0.05 mL-1kg-1hour in RS group, 0.2±0.05 mL-1kg-1hour in OS group, p=0.004). The amount of intraoperative bleeding and the number of the patients who required red blood cell transfusion were higher in the RS group than in the OS group without any statistical difference.
Postoperative outcomes and complications are shown in Table 3. The time to taking water, time to starting liquid diet, and the average length of postoperative hospital stay were similar between the two groups. The number of complications was higher in the RS group (n=5) than the OS group (n=1) without significant difference. There were three immediate complications in the RS group, consisting of two minor bile leakages and one immediate postoperative intestinal obstruction. In the OS group, there was one immediate complication of minor bile leakage. There were two 30-day complications in the RS group: one stricture of the hepaticojejunostomy site and one A-loop obstruction. There were no 30-day complications in the OS group, even though two were admitted again due to abdominal pain without any definite cause. Finally, postoperative pain scores appeared to be lower in the RS group than the OS group, although no significant differences were noted (Fig. 1).
Minimal invasive surgery (MIS) in adults has been performed extensively for various conditions. Recently, it has been applied in pediatric patients as well. In 1995, bile duct surgery using a laparoscopic approach was first demonstrated in pediatric patients.10 Several advances have been made with regard to laparoscopic techniques for hepatobiliary surgery in children; however, this approach is still not popular for many reasons: it is a highly skilled technique and much effort is required to connect a pediatric hepatic duct to the intestine with unarticulated laparoscopic instruments in a small peritoneal cavity.4,6,11 Recently, the number of parents enquiring about safe minimal invasive surgery for complex procedures for their children has grown. Meanwhile, robotic surgery has been proposed as another adjunct for pediatric minimal surgery for hepatobiliary diseases, including operations for CC.12 However, RS for pediatric CC is currently in its nascent stage, and no studies have been conducted to validate its use. To the best of our knowledge, thus far, this study is the first to compare RS and OS in order to demonstrate the validity of RS for pediatric patients undergoing CC surgery.
Laparoscopic choledochal cyst excision was the first minimally invasive surgery to be performed for pediatric choledochal cyst patients in our institution, prior to RS. However, despite the advantages of minimally invasive surgery, it has not been commonly performed due to complications associated with the procedure, its technical complexities, and difficulties with the rigid nature of the instruments and movement thereof within a limited space. Thereafter, in July 2008, RS was introduced in our institution for pediatric CC, which provided more technical advantages compared to standard laparoscopic instruments. After pediatric surgeons completed the robotic surgical training course in 2009, RS for pediatric CC was able to be performed without any open conversions. All the cases included in the present study were performed after the completion of this training course.
In the present study, patients in the RS group were significantly older than those in the OS group. We believe that this is the result of our strategy of not recommending RS in small patients with CC, because currently bulky robotic surgical systems and evolving techniques for CC would limit the application of RS in very young patients because of their small size. However, we believe that technical refinement and further miniaturization of robotic systems in the future would reduce the limiting effect of patient size in pediatric CC surgery. In the current study, the ASA physical statuses of the two groups were the same. We think that this reflects the fact that RS has been applied regardless of the patient's medical condition: we performed RS successfully regardless of whether the patient had bile peritonitis secondary to the rupture of the choledochal cyst and cholangitis. Our only exclusion criterion for RS in pediatric CC was only the body size of the patient.
The duration of surgery was longer in the RS group than in the OS group, and the mean total operation time (520±97 min) for RS group appeared to be longer than that published in the literature. However, the robotic console time was 300±81 [range 288 (185-475)] minutes and this was comparable to that reported in other studies.6,13 This difference may reflect a longer surgical duration in our early cases; this has gradually shortened with increasing experience. Another reason for the longer operation times in the RS group, compared to OS group, were related to technical aspects of initial setup, docking time, and the time taken to exchange instruments during the procedure. Another reason of our long operation times in the RS group in comparison to other studies is related to the surgeon's personal preference of applying only interrupted suturing for all hepaticojejunostomies in order to reduce the risk of anastomotic stricture. Nevertheless, given the complexity of pediatric hepatobiliary surgeries, the longer operation time is not regarded as a serious issue, as robotic surgical systems are essentially designed and utilized for precision and accuracy, not for speed.12,14,15 In addition, we think that it is also natural that the total amount of intraoperative fluid input in the RS group was larger than that in the OS group because of the longer operation time in the RS group. Meanwhile, however, the input rate of fluid necessary to maintain the same urine output was significantly smaller in the RS group. This suggests that robotic procedures may be beneficial in preventing insensible water loss and in maintaining body temperature during surgery; almost all robotic procedures were performed in a closed peritoneal cavity.
With regard to complications, minor bile leakage developed in both groups (one in the OS group and two in the RS group); however, such complications are known to occur regardless of surgical method.16,17 All of our patients with minor bile leakage were treated with short conservative medical treatment without any problems. The other more serious surgical complications such as intestinal obstruction, A-loop obstruction, and stricture at the hepaticojejunostomy site, which required additional surgical intervention, occurred during the developmental stage of the RS technique for pediatric CC.
Compared to previous studies13 that reported shorter postoperative recovery times, the current study shows that there were no significant differences in the time to dietary intake and postoperative hospital stay between the two groups. The main reason for this that the surgeon usually permitted his patients to eat only after three days following RS, even though the patients showed rapid recovery of bowel motility: he believes that three days would be the minimal safe time required for secure healing of the anastomosis even in robotic surgery. Another reason for the delay in starting food intake and delayed discharge observed in the present study may be due to one patient who developed ischemic adhesion of exteriorized bowel after RS and required a second laparotomy, and thus could only resume food intake 21 days after RS.
Research has shown that MIS in adults is associated with reduced postoperative pain.18 In pediatric patients, surgical pain and postoperative stress are usually underestimated, which may contribute to the lack of development of MIS methods for pediatric patients. However, minimal pain after surgery is also important for pediatric patients. Although the difference in pain scores between the two groups was not significant in this retrospective study, postoperative pain scores appeared to be lower in the RS group than in the OS group, which is a unique benefit of MIS, including RS. In addition, we thought that the estimation methods of postoperative pain scale score used in the present study may not provide accurate data, because the results of our data were recorded by many nurses using subjective FLACC and vNRS scales. There are certain limitations of this study. Most importantly, this is a retrospective, small, and single-center study. Also, we were unable to outline times to recovery of bowel movement in the RS group because of a lack of data thereon in medical records. As such, we perceive that the present study is mostly delineative and may present bias due to the nature of this study. Nevertheless, to date, this study is the largest to compare RS and OS and to demonstrate the validity and feasibility of RS in pediatric CC.
Despite long operation times and complications in the early stages of technical development, the results of RS for pediatric CC were comparable to open conventional surgery. We believe that with current robotic systems and surgical techniques, RS is a valid alternative for surgery in pediatric patients with CC. Nevertheless, we recognize that further prospective studies are required. We now believe that after further development of robotic surgical systems and advancement of surgical techniques, future additional prospective, controlled, and multi-center studies would support a consensus development framework for the use of RS for pediatric CC and yield more positive results.
Figures and Tables
Fig. 1
Pain scores during 36 hours after surgery. Pain scores were measured using the Faces, Legs, Activity, Cry, and Consolability (FLACC, Score 0-2) Behavior Pain Assessment Tool designed for infants and a verbal numerical rating scale for pain (0=no pain and 10=worst pain imaginable) for children every 6 hours for 36 hours post-operatively. Box plot with median (solid line), interquartile range (box), and values within 1.5 times the interquartile range (whiskers). RS, robotic surgery for pediatric choledochal cyst; OS, open surgery for pediatric choledochal cyst.

Table 1
Patient Characteristics According to Surgical Approach
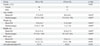
Table 2
Intraoperative Outcomes According to Surgical Approach

Table 3
Postoperative Outcomes and Complications According to Surgical Approach
References
1. Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003; 10:352–359.

2. de Vries JS, de Vries S, Aronson DC, Bosman DK, Rauws EA, Bosma A, et al. Choledochal cysts: age of presentation, symptoms, and late complications related to Todani's classification. J Pediatr Surg. 2002; 37:1568–1573.

3. Bhavsar MS, Vora HB, Giriyappa VH. Choledochal cysts: a review of literature. Saudi J Gastroenterol. 2012; 18:230–236.
4. Dawrant MJ, Najmaldin AS, Alizai NK. Robot-assisted resection of choledochal cysts and hepaticojejunostomy in children less than 10 kg. J Pediatr Surg. 2010; 45:2364–2368.

5. Akaraviputh T, Trakarnsanga A, Suksamanapun N. Robot-assisted complete excision of choledochal cyst type I, hepaticojejunostomy and extracorporeal Roux-en-y anastomosis: a case report and review literature. World J Surg Oncol. 2010; 8:87.

6. Chang EY, Hong YJ, Chang HK, Oh JT, Han SJ. Lessons and tips from the experience of pediatric robotic choledochal cyst resection. J Laparoendosc Adv Surg Tech A. 2012; 22:609–614.

7. Dangle PP, Kearns J, Anderson B, Gundeti MS. Outcomes of infants undergoing robot-assisted laparoscopic pyeloplasty compared to open repair. J Urol. 2013; 190:2221–2226.

8. Bao PQ, Mazirka PO, Watkins KT. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg. 2014; 18:682–689.

9. Diao M, Li L, Cheng W. To drain or not to drain in Roux-en-Y hepatojejunostomy for children with choledochal cysts in the laparoscopic era: a prospective randomized study. J Pediatr Surg. 2012; 47:1485–1489.

10. Farello GA, Cerofolini A, Rebonato M, Bergamaschi G, Ferrari C, Chiappetta A. Congenital choledochal cyst: video-guided laparoscopic treatment. Surg Laparosc Endosc. 1995; 5:354–358.
11. Alqahtani A, Albassam A, Zamakhshary M, Shoukri M, Altokhais T, Aljazairi A, et al. Robot-assisted pediatric surgery: how far can we go? World J Surg. 2010; 34:975–978.

12. Meehan JJ, Elliott S, Sandler A. The robotic approach to complex hepatobiliary anomalies in children: preliminary report. J Pediatr Surg. 2007; 42:2110–2114.

13. Alizai NK, Dawrant MJ, Najmaldin AS. Robot-assisted resection of choledochal cysts and hepaticojejunostomy in children. Pediatr Surg Int. 2014; 30:291–294.

14. van Haasteren G, Levine S, Hayes W. Pediatric robotic surgery: early assessment. Pediatrics. 2009; 124:1642–1649.

15. Meehan JJ, Sandler A. Pediatric robotic surgery: a single-institutional review of the first 100 consecutive cases. Surg Endosc. 2008; 22:177–182.

16. Miyano T, Yamataka A, Kato Y, Segawa O, Lane G, Takamizawa S, et al. Hepaticoenterostomy after excision of choledochal cyst in children: a 30-year experience with 180 cases. J Pediatr Surg. 1996; 31:1417–1421.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download