Abstract
Purpose
To investigate oncological outcomes based on bladder cuff excision (BCE) during radical nephroureterectomy (RNU) for upper tract urothelial carcinoma (UTUC) and to provide clinical evidence of tumor recurrence in patients without BCE.
Materials and Methods
We retrospectively collected data of 372 consecutive patients who underwent RNU at our institution from May 1989 through October 2010. After excluding some data, we reviewed 336 patients for the analysis.
Results
Of the patients who underwent RNU with BCE (n=279, 83.0%) and without BCE (n=57, 17.0%), patients without BCE had poorer cancer-specific and overall survival rates. Among 57 patients without BCE, 35 (61.4%) experienced tumor recurrence. Recurrence at the remnant ureter resulted in poor oncological outcomes compared to those in patients with bladder recurrence, but better outcomes were observed compared to recurrence at other sites. No significant predictors for tumor recurrence at the remnant ureter were identified. In patients without BCE, pathological T stage [hazard ratio (HR), 5.73] and lymphovascular invasion (HR, 3.65) were independent predictors of cancer-specific survival, whereas age (HR, 1.04), pathological T stage (HR, 5.11), and positive tumor margin (HR, 6.50) were independent predictors of overall survival.
Conclusion
Patients without BCE had poorer overall and cancer-specific survival after RNU than those with BCE. Most of these patients experienced tumor recurrence at the remnant ureter and other sites. Patients with non-organ confined UTUC after RNU without BCE may be considered for adjuvant chemotherapy with careful follow-up.
Upper urinary tract urothelial carcinoma (UTUC) is an uncommon cancer that comprises about 5% of all urological malignancies with an incidence of 0.7/100000 person-years in the United States.1,2 Although relatively rare, the incidence of UTUC seems to be increasing gradually.3,4 UTUC cells can spread via the urine and seed in underlying urothelium of the entire urinary tract.5 Because of these unique features, patients who undergo simple nephrectomy for UTUC have a high recurrence rate of 33-70% in the remaining ureteral stump.6 Radical nephroureterectomy (RNU) with bladder cuff excision (BCE), including the intramural portion and the orifice of the ipsilateral ureter, is the current standard surgical approach for UTUC.7
However, urologists have raised the question of whether they should always perform BCE during RNU since the introduction of minimal invasive surgery.8 Oncological outcome is a considerable issue for patients who undergo RNU with or without BCE. Many studies have shown poor oncological outcomes without BCE, whereas some studies have reported comparable outcomes between the two surgical approaches.9,10,11 In addition, the technical challenge should also be considered when performing RNU with BCE. For example, the surgical field may be limited in obese patients whose distal ureter is deeply located in the pelvic cavity or in patients with a history of abdominal surgery whose ureter is surrounded by adhesion tissue.12
Although many studies have provided valuable UTUC data, the detailed clinical features of recurred tumors after RNU without BCE are still unclear, including the natural course and pathological and oncological outcomes after tumor recurrence. We investigated the clinical outcomes between patients with UTUC who underwent RNU with or without BCE. We also provide pathological and oncological outcomes following salvage distal ureterectomy for recurred tumors at the retained ureteral stump. Our results offer evidence to improve the current knowledge of UTUC and its natural course.
We retrospectively collected data of 372 consecutive patients who underwent RNU for UTUC at Seoul National University Hospital (SNUH) from May 1989 through October 2010. To avoid confounding effects on oncological outcomes, we excluded 36 patients for the following reasons: incomplete tumor resection (n=4), distant metastasis at the time of RNU (n=5), no transitional cell carcinoma in the pathological findings (n=6), and lack of data (n=21). Incomplete tumor resection is defined as the unanticipated surgical consequence if the surgeons cannot completely remove the visible cancer tissues due to severe adhesion of tumors to the adjacent organs. We finally evaluated the medical records of 336 patients diagnosed with UTUC who underwent RNU with or without BCE. We did not perform BCE during RNU in 57 patients due to several reasons as follows: patients were diagnosed with renal cell carcinoma in the renal pelvis or renal parenchyma by preoperative CT or MRI and thus, they were performed radical nephrectomy and dissection of the ipsilateral ureter without BCE. Patients had aggressive tumors in the involved ureters, and the distal parts of tumors were very adhesive to adjacent organs in these cases. Although surgeons completely dissected the diseased portion of ureters harboring tumors, they failed to approach the end of the ipsilateral ureters near the bladder due to technical challenge. In one case, ipsilateral distal ureter was snapped during dissection for BCE around the outer bladder wall. These 57 patients who underwent RNU without BCE were included in subsequent analyses. The Institutional Review Board (IRB) of SNUH approved this study. Because this study was performed retrospectively, the IRB waived written informed consent from the enrolled patients.
We reviewed the following clinical and pathological data: age at RNU, gender, tumor recurrence, follow-up duration, tumor location, multifocality, and pathological findings, including pathological T stage, tumor grade, lymphovascular invasion (LVI), lymph node status, and surgical margin (SM) positivity. Surgical margin positivity, described in a pathology report, defines the visible cancer cells at the end of the resected surgical specimen under a microscopic measurement of histologic section. Pathological T stage was assessed according to the 7th edition of the American Joint Committee on Cancer staging system. Tumor grading was determined by the 1998 World Health Organization/International Society of Urologic Pathologists (WHO/ISUP) classification.
RNU was routinely performed using a double open access procedure with BCE. Radical nephrectomy and dissection of the ipsilateral ureter were performed for RNU without BCE. All surgical specimens after RNU were fixed in 10% neutral buffered formalin and embedded in a paraffin block. After sectioning at 4 mm thickness using a standard processing protocol, tissue slides were stained with hematoxylin and eosin for pathological examination. Well-experienced uro-pathologists in our hospital examined all specimens with a standard reporting protocol. After RNU for UTUC, the modality of oncological follow-up included a cystoscopy and abdominal-pelvic computed tomography with chest X-ray every 3 months during the first year and at 6-12 months thereafter.
Patient data are presented as means with standard deviations or percentages. We used the χ2 test for categorical variables and the Student's t-test for continuous variables when comparing the two groups. Oncological outcomes were represented by recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS). Survival was determined between the time of surgery and the last follow-up or death. We used the Kaplan-Meier analysis to predict the survival curve and applied the log-rank test to determine statistical significance between each survival curve.
Univariate and multivariate Cox proportional hazards regression models were used to determine significant predictors of RFS, CSS, and OS in addition to tumor recurrence at the remnant ureter. The assessed variables were gender, age, tumor location, multifocality, pathological T stage, tumor grade, LVI, lymph node status, and SM positivity. Null hypotheses of no difference were rejected if p-values <0.05 (two sided), or if the 95% confidence intervals (CIs) of the risk point estimates excluded 1. We carried out all statistical analyses using IBM SPSS Statistics 19.0 (SPSS Inc., an IBM Company, Chicago, IL, USA).
The study population consisted of 336 patients with UTUC who underwent RNU with (group 1; 83.0%, 279/336) and without BCE (group 2; 17.0%, 57/336). The baseline characteristics of the patients and the pathological stage distribution were similar between the two groups (Table 1). Tumor grade distribution pattern was not significantly different between the two groups; ≤low grade (37.6% vs. 31.8%) and high grade (62.4% vs. 68.4%) (p=0.387). Group 2 tended to show higher SM positivity (7.0%) than that of group 1 (3.9%) (p=0.238). Fig. 1 presents the Kaplan-Meier estimates for OS and CSS. The log-rank analysis revealed that group 2 showed poorer OS and CSS rates than those of group 1 (p<0.001). In the 5-year CSS- and OS-rates, group 1 showed 75.8% and 71.5%, while group 2 showed 63.9% and 57.0%, respectively.
We used a univariate Cox regression model to examine significant factors predicting oncological outcomes. No significant predictors were identified for RFS. However, we found that pathological T stage, tumor grade, LVI, lymph node status, and SM positivity were significant factors predicting CSS and further identified age, pathological T stage, tumor grade, LVI, lymph node status, and SM positivity as significant predictors of OS (Supplementary Table 1, only online). After adjusting for these variables in a multivariate analysis, we confirmed that pathological T stage [hazard ratio (HR) 5.727, 95% CI 2.082-15.752, p=0.001] and LVI (HR 3.650, 95% CI 1.468-9.072, p=0.005) were significant predictors of CSS, whereas age (HR 1.042, 95% CI 1.001-1.085, p=0.042), pathological T stage (HR 5.110, 95% CI 2.181-11.975, p<0.001), and SM positivity (HR 6.501, 95% CI 1.778-23.776, p=0.005) were significantly associated with OS (Supplementary Table 2, only online).
Of 57 patients who underwent RNU without BCE, tumor recurrence occurred in 35 (61.4%) after surgery. The recurrence sites varied as follows: remnant ureter (n=14, 40.0%), bladder (n=10, 28.6%), lymph nodes (n=3, 8.6%), multiple liver and lung (n=3, 8.6%), ureteroileal anastomotic site (n=1), duodenum (n=1), and vertebra (n=1). As shown in Fig. 2, different recurrence sites resulted in different CSS (log-rank, p<0.001). Notably, local recurrence at the remnant ureter resulted in a poorer CSS than that of bladder recurrence, whereas it showed a better CSS than other sites such as lymph nodes and distant metastasis.
We further evaluated the clinical characteristics of tumor recurrence at the remnant ureter. Of 14 patients, seven underwent salvage ureterectomy (Table 2). Four patients were treated with adjuvant chemotherapy, whereas six were not due to various reasons. Additionally, we provide the detailed information of patients who underwent salvage ureterectomy due to recurrence at the remnant ureter (Table 3). Five patients were male and two patients were female. Age at RNU was 39-74 years, and time to recurrence was 4-27 months. Of note, initial UTUC pathological stages seemed to be similar with those of recurred tumors; ≤pT1 (n=3), pT2 (n=1), and pT3 (n=3) in initial pathology, while ≤pT1 (n=3), pT2 (n=2), and pT3 (n=2) in the pathology of salvage ureterectomy. No significant predictors were identified for tumor recurrence at the remnant ureter in a univariate Cox regression model (not shown).
Although disease progression and tumor recurrence rate are high in patients with UTUC,13 clinical evidence to address these problems is lacking due to the rarity of the disease. Previous studies primarily focused on the oncological outcomes between surgical approaches, such as laparoscopic vs. open RNU, segmental ureterectomy vs. RNU, and BCE vs. non-BCE during RNU.14,15,16 Few studies have provided the clinical characteristics, oncological outcomes, and tumor recurrence rates in patients without BCE. Here, we showed data comparing baseline characteristics and oncological outcomes between patients who underwent RNU with and without BCE using homogeneous population in a single tertiary center over the last 20 years. Furthermore, we provide clinical information and oncological outcomes of patients who experienced tumor recurrence after RNU without BCE.
RNU is considered the standard surgical treatment for clinically localized UTUC.7 In the selected indications for UTUC, such as single and low-stage tumor or a solitary kidney, segmental ureterectomy can be suggested without radical resection of the entire urinary tract.7 However, this conservative approach results in high tumor recurrence of 70-90% in the remaining ureter or bladder.17 Incomplete nephroureterectomy also leads to high rates of tumor recurrence in the residual ureteral stump of 30-60%.8 Therefore, European Association of Urology (EAU) guidelines recommend that BCE should be carried out at the time of RNU to improve clinical and oncological outcomes.7 In the present study, 61.4% (35/57) of patients without BCE experienced tumor recurrence after RNU. Patients who did not undergo BCE had poorer OS and CSS rates without differences in baseline characteristics than patients who received BCE. Similarly, Lughezzani, et al.10 showed that cancer-specific mortality increased in locally advanced patients without performing BCE after examining 4210 patients with UTUC who underwent RNU with (59.2%) or without (40.8%) BCE. Hou, et al.12 reported that patients with UTUC who underwent incomplete BCE were at a higher risk for tumor recurrence than patients with complete BCE. Thus, we believe that complete resection of the bladder cuff improves oncological outcomes and minimizes the risk of tumor recurrence after RNU.
Abel, et al.18 recently reported data of 12 patients with UTUC who underwent delayed ureterectomy due to remnant ureteral recurrence after RNU. At the time of RNU, eight patients had organ-confined diseases, whereas four were diagnosed with non-organ confined diseases. After subsequent surgery for a recurred tumor at the remnant ureter, the pathological T stage did not upgrade compared to the initial pathology. In addition, no patient with initial tumor grade 2 died of UTUC regardless of the pathological findings at the time of delayed ureterectomy. In the present study, most patients among 14 patients who experienced tumor recurrence at the retained ureteral stump had advanced pathological stages (pT3 in 10, pT2 in two and pTa/1 in five). Of seven patients treated with salvage ureterectomy, patients with initial superficial tumors (pTa and pT1) have been alive regardless of pathological findings at the time of ureterectomy. Interestingly, three patients decreased their pathological T stage, whereas three other patients increased compared to the initial pathology. Due to the limited data of the recurred UTUC at the remnant ureter after surgery, our present study on the pathologic findings of recurred and primary tumors provides valuable information to understand the characteristics of recurred UTUC.
Pathological T stage is a key predictor for classifying the prognosis of patients with UTUC treated with RNU.19,20 Other potential prognostic factors are lymph node status, multifocality, LVI, carcinoma in situ, and tumor size.21,22,23 Espiritu, et al.23 identified tumor size (≥3 cm), pathological T stage (>pT2), and male gender as significant predictors of RFS in patients with UTUC after RNU, and Margulis, et al.24 reported that pathological T stage, tumor grade, lymph node status, and LVI are significant prognostic factors associated with oncological outcomes of UTUC. In our study, the multivariate analysis showed that only advanced pathological T stage (pT ≥3) was a significant predictor of OS (HR 5.110) and CSS (HR 5.727) in patients without BCE. Taken together, if patients who underwent RNU without BCE have non-organ confined UTUC, urologists should consider adjuvant systemic chemotherapy for these patients to improve their prognosis.
There are several limitations in this study. First, because the study was conducted retrospectively, the data may have been incomplete and inconsistent. Second, selection bias cannot be excluded because our institution is a tertiary referral center, and thus patients who had more aggressive tumors might be included in the study population. In fact, about 40% of patients in this study were non-organ confined state (pT ≥3), and higher proportion of non-organ confined tumors may influence the higher recurrence rate of UTUC after surgery. Third, four different surgeons performed RNU with or without BCE in our tertiary institution during the study period. Thus, the surgical technique and the therapeutic and follow-up strategies for UTUC may have been different. Finally, our study cohort was composed of a relatively small population in a single institution. In order to provide more convincing evidence, additional populations of other academic hospitals or community-based centers would be required. Nevertheless, our results offer valuable information to expand currently available knowledge on the natural course of UTUC, particularly in patients who underwent RNU without BCE.
In sum, UTUC patients who underwent RNU without BCE have a poorer CSS and OS than those with BCE. They suffered from tumor recurrence at the remnant ureter and other sites, and required salvage ureterectomy or salvage chemotherapy. Although no significant factors were identified that predicted tumor recurrence, pathological T stage was a significant predictor of CSS and OS. Therefore, if patients who undergo RNU without BCE have non-organ confined UTUC, clinicians should consider adjuvant systemic chemotherapy with careful follow-up.
Figures and Tables
Fig. 1
The Kaplan-Meier analysis for (A) OS and (B) CSS after RNU with or without BCE for treating UTUC. The log-rank test was used to determine significant differences between the two groups. RNU, radical nephroureterectomy; BCE, bladder cuff excision; UTUC, upper tract urothelial carcinoma; OS, overall survival; CSS, cancer-specific survival.

Fig. 2
The Kaplan-Meier analysis for CSS in patents who experienced tumor recurrence after RNU without BCE for treating UTUC. Four groups of patients are compared in the graph; non-recurrence, remnant ureter, bladder, and other sites. The log-rank test was used to determine significant differences between the two groups. CSS, cancer-specific survival; RNU, radical nephroureterectomy; BCE, bladder cuff excision; UTUC, upper tract urothelial carcinoma.

Table 1
Patient Characteristics
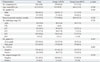
Table 2
Outcome of Patients Who Experienced Recurrence at the Remnant Ureter

Table 3
Characteristics of Patients Who Underwent Ureterectomy due to Recurrence at the Remnant Ureter

References
1. Kirkali Z, Tuzel E. Transitional cell carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol. 2003; 47:155–169.

2. Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005; 66:6 Suppl 1. 4–34.

3. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000; 164:1523–1525.

5. Booth CM, Cameron KM, Pugh RC. Urothelial carcinoma of the kidney and ureter. Br J Urol. 1980; 52:430–435.

6. McCarron JP, Mills C, Vaughn ED Jr. Tumors of the renal pelvis and ureter: current concepts and management. Semin Urol. 1983; 1:75–81.
7. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester R, Burger M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013; 63:1059–1071.

8. Srirangam SJ, van Cleynenbreugel B, van Poppel H. Laparoscopic nephroureterectomy: the distal ureteral dilemma. Adv Urol. 2009; 316807.

9. Zlotta AR. Should urologists always perform a bladder cuff resection during nephroureterectomy, and which method should they use? Eur Urol. 2010; 57:970–972.

10. Lughezzani G, Sun M, Perrotte P, Shariat SF, Jeldres C, Budaus L, et al. Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur Urol. 2010; 57:956–962.

11. Lughezzani G, Jeldres C, Isbarn H, Sun M, Shariat SF, Alasker A, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer. 2009; 45:3291–3297.

12. Hou CP, Chang PL, Chen CL, Lin YH, Tsui KH. Does adequate bladder cuff excision impact outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. Chang Gung Med J. 2011; 34:496–505.
13. Cha EK, Shariat SF, Kormaksson M, Novara G, Chromecki TF, Scherr DS, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2012; 61:818–825.

14. Jeldres C, Lughezzani G, Sun M, Isbarn H, Shariat SF, Budaus L, et al. Segmental ureterectomy can safely be performed in patients with transitional cell carcinoma of the ureter. J Urol. 2010; 183:1324–1329.

15. Fairey AS, Kassouf W, Estey E, Tanguay S, Rendon R, Bell D, et al. Comparison of oncological outcomes for open and laparoscopic radical nephroureterectomy: results from the Canadian Upper Tract Collaboration. BJU Int. 2013; 112:791–797.

16. Hung SY, Yang WC, Luo HL, Hsu CC, Chen YT, Chuang YC. Segmental ureterectomy does not compromise the oncologic outcome compared with nephroureterectomy for pure ureter cancer. Int Urol Nephrol. 2014; 46:921–926.

17. Ko R, Chew BH, Hickling DR, Razvi H, Luke PP, Chin JL, et al. Transitional-cell carcinoma recurrence rate after nephroureterectomy in patients who undergo open excision of bladder cuff v transurethral incision of the ureteral orifice. J Endourol. 2007; 21:730–734.

18. Abel EJ, Fisher MB, Matin SF, Kamat AM, Dinney CP, Grossman HB. Delayed ureterectomy after incomplete nephroureterectomy for upper tract urothelial carcinoma: pathologic findings and outcomes. Int Braz J Urol. 2013; 39:817–822.

19. Huben RP, Mounzer AM, Murphy GP. Tumor grade and stage as prognostic variables in upper tract urothelial tumors. Cancer. 1988; 62:2016–2020.

20. Oosterlinck W, Solsona E, van der Meijden AP, Sylvester R, Böhle A, Rintala E, et al. EAU guidelines on diagnosis and treatment of upper urinary tract transitional cell carcinoma. Eur Urol. 2004; 46:147–154.

21. Oikawa T, Nomura H, Kinsui H, Hamano S, Suzuki N, Tanaka M, et al. [A clinicopathological evaluation of prognostic factors of urothelial tumors of the renal pelvis and ureter]. Hinyokika Kiyo. 2001; 47:237–240.
22. Colin P, Ouzzane A, Pignot G, Ravier E, Crouzet S, Ariane MM, et al. Comparison of oncological outcomes after segmental ureterectomy or radical nephroureterectomy in urothelial carcinomas of the upper urinary tract: results from a large French multicentre study. BJU Int. 2012; 110:1134–1141.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download