Abstract
Purpose
We evaluated the incidence and risk factors of postoperative nausea and vomiting (PONV) in patients with fentanyl-based intravenous patient-controlled analgesia (IV-PCA) and single antiemetic prophylaxis of 5-hydroxytryptamine type 3 (5 HT3)-receptor antagonist after the general anesthesia.
Materials and Methods
In this retrospective study, incidence and risk factors for PONV were evaluated with fentanyl IV-PCA during postoperative 48 hours after various surgeries.
Results
Four hundred-forty patients (23%) of 1878 had showed PONV. PCA was discontinued temporarily in 268 patients (14%), mostly due to PONV (88% of 268 patients). In multivariate analysis, female, non-smoker, history of motion sickness or PONV, long duration of anesthesia (>180 min), use of desflurane and intraoperative remifentanil infusion were independent risk factors for PONV. If one, two, three, four, five, or six of these risk factors were present, the incidences of PONV were 18%, 19%, 22%, 31%, 42%, or 50%. Laparoscopic surgery and higher dose of fentanyl were not risk factors for PONV.
Conclusion
Despite antiemetic prophylaxis with 5 HT3-receptor antagonist, 23% of patients with fentanyl-based IV-PCA after general anesthesia showed PONV. Long duration of anesthesia and use of desflurane were identified as risk factors, in addition to risk factors of Apfel's score (female, non-smoker, history of motion sickness or PONV). Also, intraoperative remifentanil infusion was risk factor independent of postoperative opioid use. As the incidence of PONV was up to 50% according to the number of risk factors, risk-adapted, multimodal or combination therapy should be applied.
Postoperative nausea and vomiting (PONV) is one of the most common complications after general anesthesia, occurring up to 80%.1 Although PONV is often self-limiting, it has been cited by patients as more uncomfortable than postoperative pain.2
Perioperative opioid use has been known to be an independent risk factor for PONV. While an opioid-free pain management after surgery is ideal in terms of reducing PONV, opioids play a critical role in routine postoperative analgesic therapy. As the laparoscopic surgery with less pain is becoming popular, intravenous opioid use for postoperative analgesia is an alternative to invasive regional analgesia. Considering that most patients undergoing surgery have one or two risk factors and 20-40% of these patients are predicted to suffer from PONV,1,3 effective prophylaxis for PONV should be considered in patients with postoperative opioid use.
The 5-hydroxytryptamine type 3 (5 HT3) receptor antagonist is the mainstay of antiemetic therapy, even though there are still controversies whether risk-adapted strategy is better than liberal approach.4,5,6 In our institution, single drug therapy of 5 HT3-receptor antagonist has been administered intravenously before the end of surgery in patients with fentanyl-based intravenous patient-controlled analgesia (IV-PCA) after general anesthesia without risk assessment for PONV. However, whether single drug prophylaxis for PONV is enough especially in high risk patients, needs a reassessment.
Therefore, in this retrospective study, we evaluated the incidence and the risk factors of PONV in patients with fentanyl-based IV-PCA and single antiemetic prophylaxis with 5 HT3-receptor antagonist after the general anesthesia.
After Institutional Review Board approval (approval no: 3-2011-0238) and registration on clinicaltrials.gov (NCT0152 7890), we reviewed electronic medical records of 2039 adults with fentanyl-based IV-PCA after general anesthesia at a university hospital between May to October in 2011. The need for informed consent was waived for this study. Patients undergoing various surgeries were included in this study while patients undergoing outpatient operation were excluded. If multiple operations were performed within two days after first operation, the patients were excluded.
Single antiemetic prophylaxis with 5 HT3-receptor antagonist was done in all patients after the surgery.
Demographic and perioperative variables known to possibly be related to PONV were noted. Demographic variables included age; sex; body mass index; American Society of Anesthesiologists (ASA) physical status; history of smoking, motion sickness or PONV; previous medical history (diabetes, hypertension, radiation therapy and chemotherapy). Anesthesia- and operation-related variables were the duration of anesthesia, use of volatile anesthetics and intraoperative remifentanil infusion, emergency, laparoscopy, and operation site. Postoperative variables were PCA-related complications (headache, dizziness, sedation, pruritis, and hypotension), verbal numerical rating scale (VNRS, 0-10, 0=no symptoms; 10=worst) for pain, incidence of nausea, retching or vomiting, and requirement of rescue analgesic or antiemetic. PCA-related variables included total dose of fentanyl for two days (µg/kg), use of combined ketorolac in PCA, and discontinuation of PCA. In routine practice, PONV and pain intensity were recorded by a nurse in charge of PCA management at six times during postoperative 48 hrs (in post-anesthesia care unit, postoperative 1-6, 6-12, 12-18, 18-24, 24-48 hrs). Failure of pain management was defined as greater than 4 of VNRS score.
Values are expressed as mean±SD or the number of patients (%), as appropriate. Logistic regression models were used to identify univariate and multivariate predictors for PONV. Univariate logistic regression analysis was used first to identify possible risk factors for PONV, and the multivariate model included variables that were significant on univariate analysis. For all analyses, a p-value <0.05 was considered statistically significant. SAS 9.1 software (SAS Institute, Inc., Cary, NC, USA) was used to analyze the data.
A total of 1878 adults were included after the exclusion of 161 patients (46; incomplete data, 94; sedation due to mechanical ventilation, 21; reoperation within 48 hours after surgery). Table 1 shows patients' characteristics. Of 1878 adults, 1654 patients (89%) were maintained with volatile anesthetics. Nitrous oxide was not used in all cases. In most cases (96%), remifentanil was infused intraoperatively.
Fentanyl 16±3 ug/kg (mean±SD, range 8-29 µg/kg) was diluted in 100 mL with saline for 48 hrs PCA infusion. Fentanyl was administered at basal rate 1-2 mL/hr with bolus 0.5-1.0 mL and lock-out time 15-20 min. In most cases (93%), 4 mg of ondansetron was administered intravenously before the end of surgery and 16 mg mixed in PCA was infused during postoperative 48 hrs for the antiemetic prophylaxis. Single bolus of ramosetron (1%), granisetron (0.1%), palonosetron (3%), or ondansetron (2%) was used in other cases. Four hundred-forty patients (23%) of 1878 had showed PONV during postoperative 48 hr (Table 1). Incidence of PONV according to time after surgery was illustrated in Fig. 1. PCA was discontinued temporarily in 268 patients (14%), mostly due to PONV (88% of 268 patients).
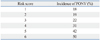
Table 2 shows the results of univariate analysis of risk factors for PONV. Laparoscopic surgery and dose of fentanyl were not associated with PONV. Sevoflurane decreased the incidence of PONV in comparison to desflurane, although use of volatile anesthetics increased the incidence of PONV (Table 2). Also, total intravenous anesthesia with propofol was not associated with PONV. Failure of postoperative pain management (defined as VNRS >4) was not associated with PONV. In the multivariate analysis, female sex, non-smoker, previous history of motion sickness or PONV, longer anesthesia than 180 min (from data mining), use of desflurane and intraoperative remifentanil infusion were identified as independent risk factors for PONV (Table 3). Table 4 shows the incidence of PONV according to the risk score based on six risk factors from our results.
In this retrospective study, the incidence of PONV in patients with fentanyl-based IV-PCA after general anesthesia was 23% despite single drug prophylaxis of 5 HT3-receptor antagonist. PCA was discontinued temporarily in 14% of total patients, mostly due to PONV. Female, non-smoker, history of motion sickness or PONV, longer anesthesia than 180 min, use of desflurane, and intraoperative infusion of remifentanil were risk factors for PONV. Postoperative pain management with opioid is associated with increasing PONV.1,3,7 The incidence of PONV has been reported to be 20-40% in patients with postoperative opioids.8,9,10,11 However, the incidence can approximate 80% in patients with multiple risk factors for PONV, if antiemetic prophylaxis is not appropriate.12,13 Compared to the incidence (34% and 45%) of previous studies used same regimen for PCA and prophylactic antiemetic,8,9 ours was slightly lower (23%), probably due to heterogeneous study cohort and antiemetic was infusion in most cases (93%) during 48 hours. However, the rate of PCA discontinuation (14%) in our study was similar to that in Song, et al.8 (10%).
Our study confirmed that female, non-smoker, history of motion sickness or PONV were independent risk factors for PONV, previously known as the components of Apfel's risk score.1,14 Also, our result demonstrated that intraoperative remifentanil infusion was a separate risk factor [odds ratio (OR) 2.82] in patients with postoperative fentanyl in PCA regimen. Volatile anesthetic increased PONV (OR 1.47, p=0.04) in accordance with other studies.7 Interestingly, desflurane increased PONV compared to sevoflurane (OR 1.42). Meta-analysis by Macario, et al.15 that showed no difference in PONV frequency between desflurane and sevoflurane. Total intravenous anesthesia with propofol and remifentanil did not reduce the incidence of PONV, although propofol is generally assumed to be antiemetic. Some reports have demonstrated that propofol with opioid did not reduce the incidence of PONV compared to balanced anesthesia with opioid.16,17,18,19 Longer duration of anesthesia was identified as a risk factor for PONV, similar to the previous study.3,20,21,22 However, the cut-off values are different among the studies and our cut-off value (180 min) is quite long compared to others. Fero, et al.23 suggested that prolonged exposure to volatile anesthetics and larger amount of opioid, which concomitant with longer duration of anesthesia or surgery, might be associated with PONV. However, whether longer use of remifentanil with short context-sensitive half time and volatile anesthetics with low solubility affects PONV in a dose-related manner is unknown.
The dose of PCA-fentanyl for 48 hrs (mean±SD; 16±3 ug/kg, range; 8-29 µg/kg) in our study was unrelated with PONV incidence, although morphine PCA has been shown to increase the incidence of PONV in a dose-related manner.24,25 Also, significant postoperative pain (VNRS >4) was not associated with PONV, in accordance with a previous prospective study.26 In the view of pain management, the dose of fentanyl in the study was not enough, because mean VNRS for pain was greater than 4 defined as failure of pain management in the early postoperative period. Within the range of our study, increasing the dose of fentanyl might reduce VNRS for pain without the increase of PONV. Laparoscopy was not associated with PONV in our study. While several studies considered laparoscopy as a risk factor for PONV, there are little data with high level of evidence.27 Instead, previous studies are in accordance with ours.21,28
Predicted incidence of PONV by Apfel's simplified risk score which is based on patients receiving volatile anesthetics without antiemetics is 10, 20, 40, 60, or 80% if 0, 1, 2, 3, or 4 of risk factors is present.1 Predicted incidence in ours is lower than that in Apfel's study, probably due to use of prophylactic antiemetic and heterogeneous study cohort including propofol anesthesia.
Combination or multimodal therapy is ideal rather than single therapy in reducing PONV. Tramer suggested 'the rule of three'; identification of patients at risk, keeping the baseline risk low, and prophylactic administration of antiemetics.29 However, recent studies suggest that compliance with these algorithms may be poor and that high-risk patients often do not receive appropriate antiemetic prophylaxis; thus, rather than risk-adapted approach, liberal and fixed combination prophylaxis could be advantageous for a larger proportion of patients.5 As the risk reduction by preventive interventions is proportion to baseline risk,30 the prophylaxis may be less advantageous in low-risk patients. The preventive intervention titration including the multiple uses of antiemetic and the avoidance of emetogenic anesthetics is appropriate and should be based on the simplified and validated risk-scoring system.
This retrospective study had some limitations. The nurse in charge of evaluating the efficacy and safety of PCA did not visit six times during each time period. The patients were asked to rate their worst nausea during each time period. This might result in bias in recalling events and an under-estimation of the incidence of PONV. Another limitation is recall bias that is originated from ignoring less severe PONV as absent.
In conclusion, PONV incidence is still 23% despite of single antiemetic prophylaxis in patient with fentanyl-based IV-PCA after general anesthesia. Combination therapy or multimodal approach should be considered. We identified female gender, non-smoker, history of motion sickness or PONV, long duration of anesthesia (>180 min), use of desflurane, and intraoperative infusion of remifentanil as risk factors for PONV in our population. We recommend that assessing for the six risk factors identified in this study would add value to the risk assessment of PONV.
Figures and Tables
Notes
References
1. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999; 91:693–700.
2. Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999; 89:652–658.

3. Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997; 52:443–449.

4. Pierre S. Risk scores for predicting postoperative nausea and vomiting are clinically useful tools and should be used in every patient: pro--'don't throw the baby out with the bathwater'. Eur J Anaesthesiol. 2011; 28:160–163.

5. Eberhart LH, Morin AM. Risk scores for predicting postoperative nausea and vomiting are clinically useful tools and should be used in every patient: con--'life is really simple, but we insist on making it complicated'. Eur J Anaesthesiol. 2011; 28:155–159.

6. Kranke P. Effective management of postoperative nausea and vomiting: let us practise what we preach! Eur J Anaesthesiol. 2011; 28:152–154.

7. Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002; 88:659–668.

8. Song JW, Park EY, Lee JG, Park YS, Kang BC, Shim YH. The effect of combining dexamethasone with ondansetron for nausea and vomiting associated with fentanyl-based intravenous patient-controlled analgesia. Anaesthesia. 2011; 66:263–267.

9. Choi YS, Shim JK, Yoon DH, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine (Phila Pa 1976). 2008; 33:E602–E606.
10. White PF, Sacan O, Nuangchamnong N, Sun T, Eng MR. The relationship between patient risk factors and early versus late postoperative emetic symptoms. Anesth Analg. 2008; 107:459–463.

11. White PF, O'Hara JF, Roberson CR, Wender RH, Candiotti KA. POST-OP Study Group. The impact of current antiemetic practices on patient outcomes: a prospective study on high-risk patients. Anesth Analg. 2008; 107:452–458.

12. Moon YE, Joo J, Kim JE, Lee Y. Anti-emetic effect of ondansetron and palonosetron in thyroidectomy: a prospective, randomized, double-blind study. Br J Anaesth. 2012; 108:417–422.

13. Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, et al. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: a prospective randomized controlled trial. Clinical article. J Neurosurg. 2009; 111:343–350.

14. Apfel CC, Roewer N. Risk assessment of postoperative nausea and vomiting. Int Anesthesiol Clin. 2003; 41:13–32.

15. Macario A, Dexter F, Lubarsky D. Meta-analysis of trials comparing postoperative recovery after anesthesia with sevoflurane or desflurane. Am J Health Syst Pharm. 2005; 62:63–68.

16. Lauta E, Abbinante C, Del Gaudio A, Aloj F, Fanelli M, de Vivo P, et al. Emergence times are similar with sevoflurane and total intravenous anesthesia: results of a multicenter RCT of patients scheduled for elective supratentorial craniotomy. J Neurosurg Anesthesiol. 2010; 22:110–118.

17. Tan T, Bhinder R, Carey M, Briggs L. Day-surgery patients anesthetized with propofol have less postoperative pain than those anesthetized with sevoflurane. Anesth Analg. 2010; 111:83–85.

18. Won YJ, Yoo JY, Chae YJ, Kim DH, Park SK, Cho HB, et al. The incidence of postoperative nausea and vomiting after thyroidectomy using three anaesthetic techniques. J Int Med Res. 2011; 39:1834–1842.

19. Höcker J, Tonner PH, Bollert P, Paris A, Scholz J, Meier-Paika C, et al. Propofol/remifentanil vs sevoflurane/remifentanil for long lasting surgical procedures: a randomised controlled trial. Anaesthesia. 2006; 61:752–757.

20. Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted. Anesthesiology. 1999; 91:109–118.

21. Choi DH, Ko JS, Ahn HJ, Kim JA. A Korean predictive model for postoperative nausea and vomiting. J Korean Med Sci. 2005; 20:811–815.

22. Kim SH, Shin YS, Oh YJ, Lee JR, Chung SC, Choi YS. Risk assessment of postoperative nausea and vomiting in the intravenous patient-controlled analgesia environment: predictive values of the Apfel's simplified risk score for identification of high-risk patients. Yonsei Med J. 2013; 54:1273–1281.

23. Fero KE, Jalota L, Hornuss C, Apfel CC. Pharmacologic management of postoperative nausea and vomiting. Expert Opin Pharmacother. 2011; 12:2283–2296.

24. Roberts GW, Bekker TB, Carlsen HH, Moffatt CH, Slattery PJ, McClure AF. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005; 101:1343–1348.

25. Anderson BJ, Ralph CJ, Stewart AW, Barber C, Holford NH. The dose-effect relationship for morphine and vomiting after day-stay tonsillectomy in children. Anaesth Intensive Care. 2000; 28:155–160.

26. Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology. 2003; 98:46–52.

28. East JM, Mitchell DI. Postoperative nausea and vomiting in laparoscopic versus open cholecystectomy at two major hospitals in Jamaica. West Indian Med J. 2009; 58:130–137.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download