Abstract
We determined the antimicrobial susceptibility of 90 clinical isolates of Stenotrophomonas maltophilia collected in 2009 at a tertiary care hospital in Korea. Trimethoprim-sulfamethoxazole, minocycline, and levofloxacin were active against most of the isolates tested. Moxifloxacin and tigecycline were also active and hold promise as therapeutic options for S. maltophilia infections.
Stenotrophomonas maltophilia is one of several opportunistic pathogens of growing significance and is the third most common nonfermentative Gram-negative bacillus that has been isolated from clinical specimens.1-3 Differentiation between S. maltophilia colonization and infection may be difficult. Despite its relatively low virulence, S. maltophilia can cause a wide variety of infections including bacteremia and pneumonia, especially in debilitated or immunocompromised patients. Risk factors for colonization and infection include mechanical ventilation, use of central venous catheters, and broad-spectrum antibiotic prophylaxis and treatment.1,4
Antimicrobial susceptibility testing methods of S. maltophilia are not clearly standardized, and there is poor correlation between disk diffusion and agar dilution results for some agents.2,5 The British Society for Antimicrobial Chemotherapy (BSAC, version 10.2, 2011) recommends disk diffusion and dilution testing for trimethoprim-sulfamethoxazole only,6 while the Clinical and Laboratory Standards Institute (CLSI) recommends dilution testing for trimethoprim-sulfamethoxazole, ceftazidime, chloramphenicol, levofloxacin, minocycline, and ticarcillin-clavulanate, and disk diffusion testing for only trimethoprim-sulfamethoxazole, levofloxacin, and minocycline.7
Infections caused by S. maltophilia are particularly difficult to manage because they show resistance to many classes of antimicrobial agents. However, few studies have focused on the susceptibility of S. maltophilia in Korea.8,9 The aim of this study was to determine the antimicrobial susceptibility of recent clinical S. maltophilia isolates from Korea.
A total of 90 non-duplicate clinical isolates of S. maltophilia were collected during 2009 from patients at a tertiary care hospital in Seoul, Korea. Sources of the isolates were respiratory specimens (n=46), urine (n=15), blood (n=11), body fluid (n=9), and pus (n=9). The species were identified using conventional methods and/or the ATB 32GN system (bioMerieux, Marcy l'Etoile, France). Antimicrobial susceptibility was tested by the CLSI agar dilution method.7
The antimicrobial agents used were trimethoprim and sulfamethoxazole (Dong Wha, Seoul, Korea), levofloxacin (Daiichi, Tokyo, Japan), minocycline (SK Chemicals Life Science, Seoul, Korea), ticarcillin and clavulanate (Dong-A, Yongin, Korea), ceftazidime, amikacin, and gentamicin (Sigma Chemical, St. Louis, MO, USA), chloramphenicol and tetracycline (Chong Kun Dang, Seoul, Korea), moxifloxacin (Bayer Korea, Seoul, Korea), tigecycline (Wyeth Research, Pearl River, NY, USA), cefepime (Bristol-Myers Squibb, Syracuse, NY, USA), imipenem (Choongwae, Seoul, Korea), piperacillin and tazobactam (Yuhan, Seoul, Korea). For the combinations of ticarcillin-clavulanate and piperacillin-tazobactam, constant amounts of clavulanate (2 µg/mL) and tazobactam (4 µg/mL) were added to ticarcillin and piperacillin, respectively. The breakpoints recommended by CLSI for S. maltophilia were applied to interpret the minimum inhibitory concentrations (MIC).7 Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as controls.
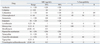
Table 1 shows the MICs of antimicrobial agents and the resistance rates of the S. maltophilia isolates tested. Among the six drugs recommended by CLSI, the relatively more active agents were trimethoprim-sulfamethoxazole, levofloxacin and minocycline.
Trimethoprim-sulfamethoxazole showed the lowest MIC90 (1 µg/mL) and the isolates showed a high susceptibility rate of 94% to this agent. All but five isolates were inhibited at ≤2 µg/mL. Resistance rates to trimethoprim-sulfamethoxazole have been reported to vary geographically,1,2,10,11 but were generally less than 20%, as was also seen in this study. However, isolates from cystic fibrosis patients and from some Asian countries, such as Taiwan and Turkey, showed high resistance rates (31.3-100%).5,12-14 Trimethoprim-sulfamethoxazole alone, or in combination with other agents, is still considered the treatment of choice for suspected or culture-proven S. maltophilia infections.
The MIC of levofloxacin ranged from 0.25 to 16 µg/mL, and the MIC50 and MIC90 were 1 and 8 µg/mL, respectively. The resistance rate was 13%, which was slightly higher than the rates of 3 to 11% seen in a previous study.1 The new fluoroquinolone, moxifloxacin, is more active in vitro than ciprofloxacin and levofloxacin.4 In this study, the MIC50 and MIC90 of moxifloxacin were 0.5 and 4 µg/mL, respectively, which were two-fold lower than those of levofloxacin. These results indicated that moxifloxacin could be suggested as an alternative to levofloxacin. Further clinical studies are necessary to evaluate the effectiveness of moxifloxacin in treating S. maltophilia infections, as there is little data published on the clinical efficacy of moxifloxacin.
In this study, all S. maltophilia isolates were inhibited by ≤4 µg/mL of minocycline, and the MIC90 was 2 µg/mL, which was 16 times lower than that of tetracycline. No isolates were resistant to minocycline. This data was similar to that of Taiwan, Brazil, Spain, and the USA.5,15-17 MICs of tigecycline ranged from 0.25 to 8 µg/mL, and MIC50 and MIC90 were 1 and 2 µg/mL, respectively, which were similar to those in other studies.1,11 The tetracycline derivatives minocycline and tigecycline have shown good in vitro activity against clinical isolates of S. maltophilia; however, there is little clinical data to support this claim.
The isolates showed low rates of susceptibility (23-43%) to ceftazidime, ticarcillin-clavulanic acid and chloramphenicol, which were slightly higher than those in other studies.1 In general, β-lactams and/or β-lactamase inhibitor combinations show little activity against S. maltophilia, because the organism has a high intrinsic resistance to most penicillins and cephalosporins, as well as to all carbapenems.
Aminoglycosides show poor activity against S. maltophilia because of high intrinsic resistance and, therefore, are not useful in monotherapy.1,4 Despite a lack of clinical trials, treatment of S. maltophilia infections with a combination of two or three antimicrobials has become current practice where trimethoprim-sulfamethoxazole therapy is contraindicated.
In conclusion, trimethoprim-sulfamethoxazole, minocycline, and levofloxacin showed good in vitro activity against S. maltophilia isolates from Korea. Moxifloxacin and tigecycline were also active and hold promise as therapeutic options for S. maltophilia.
Figures and Tables
ACKNOWLEDGEMENTS
This research was supported by the Happy Tech. Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number 20110004679).
References
1. Looney WJ, Narita M, Mühlemann K. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis. 2009. 9:312–323.
2. Sader HS, Jones RN. Antimicrobial susceptibility of uncommonly isolated non-enteric Gram-negative bacilli. Int J Antimicrob Agents. 2005. 25:95–109.

3. Jones RN, Sader HS, Beach ML. Contemporary in vitro spectrum of activity summary for antimicrobial agents tested against 18569 strains non-fermentative Gram-negative bacilli isolated in the SENTRY Antimicrobial Surveillance Program (1997-2001). Int J Antimicrob Agents. 2003. 22:551–556.

4. Nicodemo AC, Paez JI. Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur J Clin Microbiol Infect Dis. 2007. 26:229–237.

5. Wang WS, Liu CP, Lee CM, Huang FY. Stenotrophomonas maltophilia bacteremia in adults: four years' experience in a medical center in northern Taiwan. J Microbiol Immunol Infect. 2004. 37:359–365.
6. BSAC Methods for antimicrobial susceptibility testing. access on June 2011. Available at http://www.bsac.org.uk/Susceptibility+Testing/GUIDELINES+Standardized+Disc+Susceptibility+Testing+Method.
7. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement, M100-S21. 2011. Wanye, PA: Clinical and Laboratory Standards Institute.
8. Song JH, Sung JY, Kwon KC, Park JW, Cho HH, Shin SY, et al. Analysis of acquired resistance genes in Stenotrophomonas maltophilia. Korean J Lab Med. 2010. 30:295–300.

9. Seol SY, Jang KS, Jeong OG, Cho ER, Kim NH, Yu HS, et al. Antimicrobial resistance and molecular epidemiologic characteristics of Stenotrophomonas maltophilia isolated from clinical specimens. J Korean Soc Microbiol. 2000. 35:239–250.
10. Livermore DM, Hope R, Brick G, Lillie M, Reynolds R. BSAC Working Parties on Resistance Surveillance. Non-susceptibility trends among Pseudomonas aeruginosa and other non-fermentative Gram-negative bacteria from bacteraemias in the UK and Ireland, 2001-06. J Antimicrob Chemother. 2008. 62:Suppl 2. ii55–ii63.
11. Farrell DJ, Sader HS, Jones RN. Antimicrobial susceptibilities of a worldwide collection of Stenotrophomonas maltophilia isolates tested against tigecycline and agents commonly used for S. maltophilia infections. Antimicrob Agents Chemother. 2010. 54:2735–2737.

12. Cantón R, Valdezate S, Vindel A, Sánchez Del Saz B, Maíz L, Baquero F. Antimicrobial susceptibility profile of molecular typed cystic fibrosis Stenotrophomonas maltophilia isolates and differences with noncystic fibrosis isolates. Pediatr Pulmonol. 2003. 35:99–107.

13. Gülmez D, Hascelik G. Stenotrophomonas maltophilia: antimicrobial resistance and molecular typing of an emerging pathogen in a Turkish university hospital. Clin Microbiol Infect. 2005. 11:880–886.

14. Valenza G, Tappe D, Turnwald D, Frosch M, König C, Hebestreit H, et al. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J Cyst Fibros. 2008. 7:123–127.

15. Galles AC, Jones RN, Sader HS. Antimicrobial susceptibility profile of contemporary clinical strains of Stenotrophomonas maltophilia isolates: can moxifloxacin activity be predicted by levofloxacin MIC results? J Chemother. 2008. 20:38–42.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download