Abstract
We report on a patient with a partial deletion on the short arm of chromosome 18 (del 18p), who presented with dysmorphic features and delayed developmental milestones as well as with a patent ductus arteriosus (PDA) and pulmonary valve stenosis (PS). Several forms of congenital heart disease (CHD) are found in about 10% of patients with del (18p), but coexisting PDA and PS have not been reported. Del (18p) must be considered in patients with characteristic phenotypic abnormalities and congenital heart disease, including a combination of PDA and PS.
The del 18p syndrome is the second most frequent autosomal deletion syndrome. Since the first report of the del 18p in 1963, more than 150 cases have been reported.1,2 There is a broad variability of phenotypic features. Short stature, facial dysmorphism, and skeletal and cardiac anomalies are commonly seen. Congenital heart disease (CHD) is estimated to be present in about 10% of these patients.3,4 Here we report a case of patent ductus arteriosus (PDA) in combination with pulmonary valve stenosis (PS), dysmorphic features, and mental and growth retardation in a boy with del 18p.
The boy is the first and only child born to healthy, non-consanguineous parents. At birth, the mother was 27 years old, and the father was 28 years old. No exposure to alcohol, nicotine, drugs or human teratogens during pregnancy was reported. The baby was delivered full-term at 40 weeks of gestational age with a birth weight of 3500g, normal Apgar scores and no signs of asphyxia.
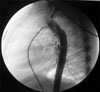
He was referred to our hospital with a heart murmur at the age of two years old. Echocardiography showed a PDA and a mild PS. The pressure gradient from the right ventricle to the pulmonary artery was measured as 18 mmHg. Dysmorphic features were not obvious at that time. The child underwent transcatheter closure of the PDA. The narrowest diameter of the PDA was 2.7 mm on selective angiography (Fig. 1), and a 4 × 6 cm Amplatzer PDA occluder (AGA Medical) was placed successfully (Fig. 2). Pressures were 42/1mmHg in the right ventricle and 25/13mmHg in the pulmonary artery with a resulting systolic pressure gradient of 17 mmHg, correlating well with the echocardiographic measurement and indicating mild PS.
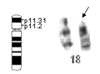
At follow-up two years after PDA closure, the boy had developed dysmorphic features, including a full face, low-set ears, a depressed nasal bridge, upturned nostrils, a long philtrum, irregular carious teeth and a high palate. His development was delayed. Physical examination showed a body height of 94 cm, a weight of 12.5 kg and an occipito-frontal circumference of 48 cm. At 35 months of age, the child could only speak using single words. He also had an overly friendly personality. The cranial CT scan showed no abnormal findings. Tested with the Wechsler Preschool and Primary Scale of Intelligence (WPPSI),5 a scale that has already been normalized in China, his overall IQ score was 78. His Verbal IQ and Performance IQ scores were 75 and 85, respectively. Initially, the child was suspected to have Williams syndrome because of his dysmorphic features, symptoms of congenital heart disease and his very outgoing and overly friendly behavior. Two-color fluorescence in situ hybridization (FISH) using an Elastin gene locus specific probe (32190041, Vysis Inc., Downers Grove, IL, USA) on 7q11.23 ruled out Williams syndrome. Then chromosome analysis using standard Giemsa-banding methods revealed a 46, XY, del (18) (p11.3) karyotype (Fig. 3), while both parents had normal chromosomes.
Del 18p is one of the most common autosomal deletion syndromes. It is characterized by mild-to-severe mental retardation. Among the most consistent features are growth deficiency, microcephaly (29%), facial dysmorphism, epicanthus (40%), ptosis (38%), microgenia, low nasal bridge, hypertelorism (41%), a wide mouth, and large protruding ears. Teeth are often misaligned and show carious defects. Additional findings include a webbed neck, widely spaced nipples, clinodactyly of the fifth finger, syndactyly, and strabismus. The most important congenital malformation found in patients with the del 18p syndrome is holoprosencephaly (10 - 12%). Other congenital malformations include cardiac vitia (10%) and genital anomalies (18%).6,7 The wide variability of phenotypic manifestations makes its diagnosis difficult. The positive findings in our patient included: dysmorphic features, growth deficiency, speech delay and cardiac abnormalities. The dysmorphic features were not very obvious during the first hospitalization at two years of age, but became striking during follow-up visits when the child was four years old. There may be a tendency of the dysmorphic features to become more apparent with age.
Patients with complete or partial deletions on the short arm of chromosome 18 suffer from congenital heart disease in about 9% to10% of the cases.3,4,6,8 In a review of the literature, Digilio et al.4 collected 12 cases with CHD and del 18p, which included different entities such as PDA, ventricular septal defect, aortic stenosis, and dextrocardia with situs inversus, among others. Isolated PDAs were diagnosed in two patients. Complete situs inversus associated with subaortic ventricular septal defect and pulmonary stenosis was present in one patient and d-Transposition of the great arteries complicated with pulmonary stenosis was diagnosed in another patient. None of the reported patients had a combination of PDA and PS.
PDA is a common congenital heart disease that results when the ductus arteriosus, a muscular artery, fails to remodel and close after birth. Familial cases of PDA have been recorded, but genetic causes have not been determined. Several chromosomal abnormalities are associated with a PDA.9,10 In older infants and children, options for closure of PDA include surgery or transcatheter closure with a PDA occluder or coil. The association of PS and PDA is uncommonly found in the clinical practice of pediatric cardiology. On review of the medical literature this represents the first reported case of PDA and PS in a patient with del 18p.
Although different neurological symptoms were described in the context of deletions of 18p, including mental retardation, dystonia, seizures, tremor, and chorea,11 it is not clear what sort of behavioral symptoms are associated with this particular chromosome disorder. Our patient achieved an overall IQ score of 78, and he also exhibited an outgoing and overly friendly personality. The test results revealed borderline mental retardation. Wester et al.12 studied a genotype-phenotype correlation of seven cases with de novo 18p deletions and found that there might be a critical region between 18p11.1 and 18p11.21. However, further investigations are needed to elucidate the relationship between 18p deletions and the resulting behavioral and developmental phenotype.
Figures and Tables
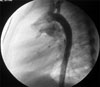
Fig. 1
An aortography in the lateral projection showed the shape and size of the PDA. PDA, patent ductus arteriosus.
References
1. Kim YM, Cho EH, Kim JM, Lee MH, Park SY, Ryu HM. Del (18p) syndrome with increased nuchal translucency in prenatal diagnosis. Prenat Diagn. 2004. 24:161–164.

2. Schaub RL, Reveles XT, Baillargeon J, Leach RJ, Cody JD. Molecular characterization of 18p deletions: evidence for a breakpoint cluster. Genet Med. 2002. 4:15–19.

3. Vasquez JC, Rabah R, Delius RE, Walters HL. Hypoplastic left heart syndrome with intact atrial septum associated with deletion of the short arm of chromosome 18. Cardiovasc Pathol. 2003. 12:102–104.

4. Digilio MC, Marino B, Giannotti A, Di Donato R, Dallapiccola B. Heterotaxy with left atrial isomerism in a patient with deletion 18p. Am J Med Genet. 2000. 94:198–200.

5. Gong YX, Dai XY. Handbook of Wechsler Preschool and Primary Scale of Intelligence in China. 1992. Changsha: Hunan map publishing company.
6. Strenge S, Froster UG. Diaphragmatic hernia in 18p-syndrome. Am J Med Genet A. 2004. 125A:97–99.
7. Brenk CH, Prott EC, Trost D, Hoischen A, Walldorf C, Radlwimmer B, et al. Towards mapping phenotypical traits in 18p-syndrome by array-based comparative genomic hybridisation and fluorescent in situ hybridisation. Eur J Hum Genet. 2007. 15:35–44.

8. Movahhedian HR, Kane HA, Borgaonkar D, McDermott M, Septimus S. Heart disease associated with deletion of the short arm of chromosome 18. Del Med J. 1991. 63:285–289.
9. Slavotinek A, Clayton-Smith J, Super M. Familial patent ductus arteriosus: a further case of CHAR syndrome. Am J Med Genet. 1997. 71:229–232.

10. Mani A, Radhakrishnan J, Farhi A, Carew KS, Warnes CA, Nelson-Williams C, et al. Syndromic patent ductus arteriosus: evidence for haploinsufficient TFAP2B mutations and identification of a linked sleep disorder. Proc Natl Acad Sci U S A. 2005. 102:2975–2979.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download