Abstract
Purpose
To evaluate the safety and efficiency of the Ultrasound (US)-guided large needle core biopsy of axilla lymph nodes.
Materials and Methods
From March 2004 to September 2005, 31 patients underwent the US-guided core biopsy for axilla lymph nodes. Twenty five lesions out of 31 were detected during breast US, and 6 of 31 cases were palpable. Lymph nodes were classified based on their shape and cortical morphology. The core biopsy of axilla lymph nodes was performed on suspicious lymph nodes found during breast ultrasonography to find out whether the patients had a history of breast cancer or not. Among the 31 patients, 16 patients were associated with breast cancer. The lesion sizes varied from 0.6 cm to 3.3 cm (mean = 1.59 ± 0.76 cm). US-guided core biopsies were performed with 14 G needles with an automated biopsy gun. Total 3 or 5 specimens were obtained.
Results
Among the 31 cases of axilla lymph nodes core biopsies, 11 cases showed malignant pathology. Seven out of 11 cases were metastatic lymph nodes from breast cancer; 2 cases were from primary unknown and 2 cases from lymphomas. On the other hand, 20 histopathologic results of axilla lesions were benign: subacute necrotizing lymphadenitis (n = 2), dermatopathic lymphadenitis (n = 1), reactive hyperplasia (n = 10) and free of carcinoma (n = 7).
Pathologic lesions in the axilla develop in a wide range of disease. Among them, lymph node abnormality is the most common pathologic finding. The causes of the lymph node abnormality also vary, ranging from benign inflammatory disorders to malignant reasons.1 Most cases of lymphadenopathies detected through imaging examinations are not palpable, and pretreatment pathologic diagnoses are demanded. Fine needle aspiration cytology under the ultrasound guidance is commonly employed and has been reported to be the easiest and accurate method.2-4 Jaffer et al. reported the sensitivity and the specificity of the ultrasound-guided fine-needle aspiration cytology (US-guided FNAC) as high as 100%,5 and another report presented that the overall sensitivity of the US-guided FNAC was 86.4%, the specificity was 100%, and the diagnostic accuracy was 79%.3 However, the false negative rate of FNAC was 11.6%, and 8 of 38 patients (21.0%) who did not receive preoperative chemotherapy because of false negative rate.3 Biopsies with 18-gauge needle of the small series have been reported, and several studies have recently reported about the ultrasound-guided larger 14-gauge needle core biopsy of the axilla lesions.6,7 An imaging-guided core biopsy has higher sensitivity than FNAC for assessing breast lesions, and the trend would be similar to the US-guided core biopsy of the axilla. The purpose of this study was to investigate the usefulness and safety of the US-guided core biopsy of axilla lesions and to evaluate the benefit of preoperative diagnosis of the nodal staging in breast cancers.
From March 2004 to September 2005, 31 patients with suspicious lymph nodes in the axilla, visible in ultrasound, underwent the US-guided core biopsy. A waiver of informed consent from institutional review board before our study was obtained. All patients underwent clinical examinations and mammogram before their ultrasound examinations. The mean age of patients was 45 ± 15.7 (from 31 to 64 years). Among 31 patients, 6 patients complained of a palpable mass in the axilla, and suspicious lymph nodes were incidentally found in the remaining 25 patients during the breast ultrasound. Abnormal Lymph nodes were categorized according to their shape and the morphology of the cortex and hilum as follows: 1) there was the length to width ratio (l/w) with less than 1.5, since l/w higher than 2.0 indicates that the node is benign;6 2) The cortex of the nodes was concentrically or eccentrically thickened to more than 2 mm;6 3) Compression of the hilus and especially the absence of the hilus are thought to be highly suggestive of malignancy. If the axillary lymph nodes exhibited any of abnormal findings mentioned above, they were defined as suspicious.
Sixteen patients were associated with breast cancer, and 15 other patients had no lesion in the breast. Among 16 breast cancer patients, 13 cases had a previous history of breast cancer. Their follow-up breast ultrasound revealed suspicious lymph nodes in the axilla without breast lesions. Seven out of 13 cases were contralateral lymph node, and 6 cases were ipsilateral lymph node in the axilla. Remaining 3 out of 16 patients were diagnosed of BI-RADS (Breast Imaging Reporting and Data System) category 5 lesions in the breast and suspicious lymph nodes in the axilla at the same tim: Those were seen at ipsilateral in one case and contralateral in two cases. The TNM stages of the breast cancer patients were stage I (n = 0), IIA (n = 2), IIB (n = 6), IIIA (n = 3), IIIB (n = 2), and IV (n = 0) in previously diagnosed patients, and stage IIB (n = 2) and IIIA (n = 1) in currently diagnosed patients.
Ultrasound scanning of the axilla and breast was carried out with a 10 - 12 MHz transducer with HDI 5000 machine (Advanced Technology Laboratories, Bothell, WA, USA).
The core biopsies of nonpalpable axilla lymph nodes were performed on suspicious axilla lymph nodes which were found during breast ultrasound. The contralateral or ipsilateral suspicious axilla lymph nodes in patients with ACR BI-RADS category 4 or 5 lesion in their breast and those patients whose breast cancer had previously been treated were also indicated. The lesion size was 0.6 cm to 3.3 cm (mean = 1.59 ± 0.76 cm). All biopsies were ultrasound-guided with a free hand approach. Fourteen-gauge (2.1 mm) needles were used with an automatic biopsy gun, and 15 mm shooting was selected (Bard-Magnum Biopsy Instrument, Covington, GA, USA). The number of needle pass was 3 to 5. For the evaluation of any post-procedural complications at the site of the biopsy, all patients underwent clinical examinations a few days after the biopsy.
For the patients who underwent axillary dissection (n = 4), the number and size of the dissected and metastatic nodes were reported. The close follow-up was conducted with ultrasound in patients treated with chemotheraphy or radiotheraphy. The cases of benign pathology were examined by ultrasound over the next 2 years.
Among 31 cases of axilla lymph nodes biopsy, 11 cases showed malignant and 20 cases benign pathology. Seven of the 11 malignant nodes were metastatic lymph nodes from breast cancer; 2 were from unknown primary, and remaining 2 from lymphoma. Twenty benign histopathologic results were as follows: subacute necrotizing lymphadenitis in 2, dermatopathic lymphadenitis in 1, and reactive hyperplasia in 10 and free of carcinomas in 7 (Table 1). Among them, 8 patients had a previous history of breast cancer, and one patient was diagnosed with breast cancer at the current examination. There was no complication during and after the core biopsy procedure, and plenty of specimens were obtained even in a small lesion less than 1 cm in size.
Out of 7 patients with breast cancer, 5 patients had a previous history of breast cancer. Only one patient showed an ipsilateral lymph node metastasis and 4 patients demonstrated a contralateral lymph nodes metastasis. All 5 patients received chemotheraphy, and 2 patients had a systemic metastasis, such as pericardium and supraclavicular lymph nodes metastases or a lung metastasis. One patient underwent contralateral axillary dissection, and metastasis in 5 out of 19 dissected lymph nodes was confirmed (Fig. 1).
Two of 7 patients were diagnosed with both breast cancer and metastatic lymph node by core biopsy. One of the 2 cases was crossed axillary metastasis, i.e., breast cancer in the right breast and a metastatic lymph node in the left axilla. Hence, she was taken for a modified radical mastectomy of the right breast and the contralateral axillay dissection and radiotherapy. The pathologic specimen showed positive in 14 of 19 lymph nodes. The other one patient received the modified radical mastectomy and the ipsilateral axillay dissection, which revealed 3 metastatic of 18 lymph nodes.
Two out of the 11 malignant pathologic results revealed metastatic lymph nodes (Fig. 2), however, primary site could not be identified through the mammogram, whole breast ultrasound, MRI and whole body PET. Thus, they underwent the axillary dissection and ipsilateral breast radiotherapy, and a close follow-up was performed for over 2 years.
Remaining 2 patients were diagnosed with Hodgkin's lymphoma and B-cell lymphoma, respectively. They were treated with chemotheraphy, and the outcomes were highly satisfactory.
In the cases of chemotheraphy or radiotheraphy (n = 7), their follow-up ultrasound revealed that the pathologic lymph nodes were reduced in size and showed thinning of the cortex (Fig. 3). The mean of maximum diameters of lymph nodes before chemotherapy was 1.37 ± 1.1 cm and it was reduced to 0.72 ± 1.4 cm after the chemotherapy.
The percutaneous image-guided core biopsy increasingly becomes an alternative to a surgical biopsy for the histologic assessment of breast lesions.8 Guidance for the percutaneous biopsy is provided by stereotaxis or ultrasound. Especially, the ultrasound-guided 14-gauge automated core biopsy is safe, fast, accurate, and cost effective. Advantages of ultrasound as a guidance modality for the percutaneous biopsy include lack of ionizing radiation, use of nondedicated equipment, accessibility of all areas of the breast and the axilla, real- time visualization of the needle, multidirectional sampling, and low cost.9 The only disadvantage of the ultrasound guidance is that the lesion must be sonographically evident for implementing the ultrasound-guided biopsy. Therefore, we consider that the ultrasound guided axilla lymph nodes core biopsy can also be an alternative to the surgical biopsy for the histologic evaluation of axilla lesions.
The axillary nodal status is a very important factor in the preoperative staging in breast cancer patients, and it is also crucial in the pre-op decision to perform a sentinel node biopsy or axillary lymph node dissection. In a majority of cases, US-guided FNAC has been an initial procedure to detect the lymph node metastasis in the axilla. However, the US-guided FNAC exhibits relatively high false negative rate (11.6% to 21.0%),3 and this is most probably due to inadequate volume sampling, fibrosis, inflammatory reactions, and previous radiation therapies.10 Hence, several authors6,7 tried to replace the US-guided FNAC of axilla lymph nodes with the large needle core biopsy using a gun in the preoperative staging of breast cancer patients. To our best knowledge, previous studies performed the core biopsy by using 16-gauge or 18-gauge needles, and there are a few reports about the 14-gauge needle core biopsy of the axilla lymph node.6,11,12 In the case of the breast core biopsy, we routinely implement the 14-gauge needle for sampling more specimens. However, it is undoubtful whether the more specimens we obtain, the more precise diagnosis is obtainable. In our series, we acquired 11 cases of malignant pathology in the core biopsy. Except 4 patients who needed the axilla dissection for clearing the breast cancer treatment, the axillary dissection was unnecessary for remaining 7 patients. In cases of benign pathology in the axilla lymph node core biopsy, there was no case to suggest false negative results such as the progression of the lymph node size or the change of the morphology in the ultrasound follow-up. Therefore, we postulate that the ultrasound follow-up of pathologically confirmed benign lymph nodes in the core biopsy is a satisfactory without any additional imaging study or a further surgical intervention.
Various complications of the percutaneous core biopsy such as hemorrhage or infection have been reported. However, there were no cases of non-diagnostic specimens and no complications in our 31 patients who had received the large needle gun biopsy (14G) of axilla lymph nodes. Nevertheless, a special care should be taken for reducing unwanted complications by fully acknowledging detailed anatomy including vessels and nerves in the axilla. The nerves are not usually visible on ultrasound, while the axillary vein and artery can easily be recognized. Since the major nerves lie between the vein and artery, their path can be presumed through imaging the vessels.7 Lymph nodes, on the other hand, lie more superficially than the neurovascular structures. Thus, proper positioning of patients and perfect guiding by ultrasound are highly important to avoid traumatic injuries during the large needle core biopsy of the axilla.
In conclusion, the US-guided large needle (14G) core biopsy of the axilla lesion is safe and effective for the pathological evaluation. The core biopsy could easily be employed if suspicious lymph nodes or mass lesions were found in the axilla. Especially in breast cancer patients, the core biopsy of the axilla lymph node could be an effective alternative to surgical biopsy for the histological diagnosis.
Figures and Tables
Fig. 1
A 51-year-old woman with a metastatic lymph node in the right axilla. (A) The mediolateral oblique view of mammography shows an enlarged high density lymph node in the right axilla without mass or microcalcifications in the right breast. (B) Ultrasonography revealed a 1.48 × 0.71 cm sized hypoechoic lymph node in the right axilla, showing loss of a fatty hilum. (C) The ultrasound guided 14-gauge automated core biopsy was performed at the axilla lymph node, and the real time visualization of the needle was achievable. (D) A large needle (14-gauge) lymph node specimen (H&E stain, ×20) shows the cores of metastatic carcinoma occupying almost the entire biopsied lymph node tissue. (E) Photomicrograph shows clusters of infiltrating metastatic carcinoma cells on the right side of view (H&E, ×400).
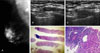
Fig. 2
A 50-year-old woman with contralateral metastatic axillary lymph node after right modified radical mastectomy 2 years ago. (A) Single enlarged axillary lymph node with eccentric cortical thickening is noted in the left axilla. The size of the lymph node is 2.0 × 1.5 cm. (B) The ultrasonography guided large needle core biopsy shows correct sampling for the eccentric lymph node cortex. (C) The dissected axillary lymph node shows infiltration of the metastatic carcinoma (lower left, H&E, ×100). The lymph node parenchyma shows partial fibrosis due to previous chemotherapy. (D) The core shows infiltration of the metastatic carcinoma (H&E, ×100).
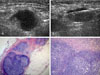
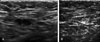
Fig. 3
A 40-year-old woman with left modified radical mastectomy 5.5 years ago. (A) Post-op follow up ultrasonography shows eccentric cortical thickening of the contralateral axillary lymph node, which was proved to be the metastatic lymph node by the core biopsy using 14 gauge large needle. (B) After chemotherapy, the size of the metastatic axillary lymph node was reduced from 15 to 8 mm in the largest dimension.
References
2. de Kanter AY, van Eijck CH, van Geel AN, Kruijt RH, Henzen SC, Paul MA, et al. Multicentre study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br J Surg. 1999. 86:1459–1462.

3. Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002. 95:982–988.

4. Oruwari JU, Chung MA, Koelliker S, Steinhoff MM, Cady B. Axillary staging using ultrasound-guided fine needle aspiration biopsy in locally advanced breast cancer. Am J Surg. 2002. 184:307–309.

5. Jaffer S, Zakowski M. Fine-needle aspiration biopsy of axillary lymph nodes. Diagn Cytopathol. 2002. 26:69–74.

6. Damera A, Evans AJ, Cornford EJ, Wilson AR, Burrell HC, James JJ, et al. Diagnosis of axillary nodal metastases by ultrasound-guided core biopsy in primary operable breast cancer. Br J Cancer. 2003. 89:1310–1313.

7. Abdsaleh S, Azavedo E, Lindgren PG. Ultrasound-guided large needle core biopsy of axilla. Acta Radiol. 2004. 45:193–196.

8. Liberman L. Clinical management issues in percutaneous core breast biopsy. Radiol Clin North Am. 2000. 38:791–807.

9. Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002. 40:483–500. vi

10. Hsu C, Leung BS, Lau SK, Sham JS, Choy D, Engzell U. Efficacy of fine-needle aspiration and sampling of lymph nodes in 1,484 Chinese patients. Diagn Cytopathol. 1990. 6:154–159.

11. Susini T, Nori J, Vanzi E, Livi L, Pecchioni S, Bazzocchi N, et al. Axillary ultrasound scanning in the follow-up of breast cancer patients undergoing sentinel node biopsy. Breast. 2007. 16:190–196.

12. Nori J, Bazzocchi M, Boeri C, Vanzi E, Nori Bufalini F, Mangialavori G, et al. Role of axillary lymph node ultrasound and large core biopsy in the preoperative assessment of patients selected for sentinel node biopsy. Radiol Med (Torino). 2005. 109:330–344.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download