Abstract
Purpose
The author presents imaging findings of patients that underwent partial resection of the breast followed by absorbable mesh implantation.
Materials and Methods
Ultrasonographic (n = 18) and mammographic (n = 11) images of patients that had undergone absorbable mesh implantation after breast partial resection were reviewed retrospectively. Sequential changes of the lesions were analyzed in follow-up ultrasonographic examinations, focusing on the change of the size and pattern of the lesion. The presence of a mass, asymmetry, focal asymmetry, architectural distortion, and calcification were evaluated by mammography. Pathologic findings of the implanted mesh in available cases were analyzed.
Results
Ultrasonograms revealed a well-encapsulated anechoic lesion with (pattern 1, n = 11) or without (pattern 2, n = 5) internal isoechoic nodular portion, and a hyperechoic mass-like lesion without anechoic portion (pattern 3, n = 2). The mean length of the longest diameter decreased gradually as determined in follow-up examinations (3 months, 6.12 ± 2.599 cm; 6 months, 5.08 ± 2.105 cm; 12 months, 3.26 ± 2.206 cm). In mammograms, a mass (n = 4) was noted at the surgical site and focal asymmetry, overlapping with the postoperative change, was seen in the remaining seven cases. Pathologic findings of two cases revealed foreign body reaction.
Conclusion
Ultrasonography of the patients that underwent breast partial resection followed by absorbable mesh implantation showed a well-encapsulated cyst at the surgical site that gradually decreased in follow-up examinations. Adjunctive ultrasonography combined with mammography would be recommended in postoperative follow-up examinations.
Breast conserving surgery is now a standard surgical treatment for women with relatively small breast cancers.1 Although one can preserve the integrity of the breast by the use of conserving surgery, poor patient satisfaction due to breast deformity is still a continuing problem. Breast reconstruction using various tissue flaps or artificial prostheses has been performed to maintain the appearance of the breast. However, these are complex procedures and require extensive experience in plastic surgery.
In our hospital, a breast surgeon has used absorbable mesh materials for breast implantation to minimize breast deformity in patients that have undergone breast partial resection due to a small breast cancer or a high risk benign condition since 2005. The one-step operation was performed by breast partial resection using endoscopic quadrantectomy or wide local excision, followed by immediate absorbable mesh implantation using polyglactin or polyglycolic acid mesh. A sheet of absorbable mesh (Fig. 1), folded in a fan shape and wrapped with oxidized regenerated cellulose to avoid tissue adhesion was inserted into the surgical site.
The author presented early postoperative ultrasonographic findings of the implanted absorbable mesh in patients with breast partial resection.2 Follow-up ultrasonography, mammography, and pathologic evaluation were performed afterward. The aim of the present study is to present the follow-up ultrasonographic and mammographic findings of implanted absorbable mesh in patients that underwent breast partial resection.
Between May 2005 and April 2006, a total of 22 women were treated consecutively in our hospital with breast partial resection and absorbable mesh implantation to minimize breast deformity. Twenty patients underwent postoperative ultrasonographic examinations. However, two of the patients that were initially examined more than 6 months after surgery were excluded from the study. Eighteen patients (age range, 30 - 61 years; mean age, 43 years) who had undergone the initial postoperative ultrasonography about 3 months after surgery were examined with follow-up ultrasonography in 6 and/or 12 months. Of the 18 study patients, 11 had postoperative mammograms. Two patients underwent pathologic examinations about 1 and 16 months after mesh implantation, respectively.
The ultrasonography was performed with an IU-22 (Philips Medical Systems, Bothell, WA, USA) and a HDI-5000 (ATL, Bothell, WA, USA) instruments using 12 - 17 and 12 MHz linear array transducers, respectively. Gray scale and color Doppler scans were obtained, and a panoramic scan was sometimes necessary to obtain because of the large size of a lesion.
Initial breast ultrasonographic examinations were performed about 3 months (interval range, 29 - 133 days; mean, 92 days) after surgery in all patients. Of the 18 study patients, follow-up examinations were performed at least one time, but the number of examinations and the interval period between surgery and follow-up examinations varied (Table 1). Seven of 18 patients underwent two additional follow-up ultrasound examinations about 6 and 12 months after surgery. Six of 18 patients underwent a follow-up examination about 6 months after surgery. The remaining five patients underwent a follow-up examination about 12 months after surgery.
Mammography was performed with either of two conventional screen-film systems: Senographe DMR (General Electric Medical systems, Milwaukee, Wisconsin, USA) and M-IV (Lorad, Danbury, Connecticut, USA). Mammography was not performed during early postoperative period until 3 months due to a concern of rupturing the implanted mesh. Postoperative mammography was performed for 11 cases, including 7 cases of invasive ductal carcinoma (IDC), 2 cases of ductal carcinoma in situ (DCIS), and 2 cases of atypical ductal hyperplasia (ADH). The interval between surgery and the initial postoperative mammography varied from 3 to 12 months (interval range, 92 - 350 days; mean, 191 days) and follow-up examinations were performed with a 6 months-interval in 9 cancer patients. The total number of mammographic examinations varied (range, 1 - 4 times; mean, 2.18 times).
The author, who has 5-years-experience of breast imaging, retrospectively reviewed the ultrasonographic and mammographic images with knowledge of all associated clinical, surgical, and histopathologic information.
Ultrasonographic findings of the implanted absorbable mesh were classified into three patterns based on the presence of capsule, cyst, and the internal content of the cyst: pattern 1) a well-encapsulated anechoic lesion with internal isoechoic nodular portion; pattern 2) a well-encapsulated anechoic lesion without internal isoechoic nodular portion; pattern 3) a hyperechoic mass-like lesion without anechoic portion. The size of the lesion was measured in transverse or panoramic scan and marked as the longest × shortest diameter. Sequential changes of the lesions were analyzed in follow-up ultrasonographic examinations, focusing on the change of the size and pattern of the lesion.
The presence of a mass, asymmetry, focal asymmetry, architectural distortion, and calcification were analyzed by mammography. The mammographic appearance of each lesion was classified by using the BI-RADS lexicon. In follow-up examinations, the interval change as compared with a previous study was analyzed.
A sequential change of lesion size was analyzed by comparing the data of 3 months with that of 6 and 12 months after surgery. Statistical analysis was preformed for seven cases that had 3, 6, and 12 months-period follow-up data. It was performed by use of SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA) software and general linear models (GLM)-repeated measures. P value was considered statistically significant at the p < 0.05 level.
Patient characteristics, ultrasonographic findings of all cases in the initial and follow-up examinations, and mammographic findings are summarized in Table 1. In the initial ultrasonographic examinations about 3 months after surgery, 11 cases showed pattern 1, 5 cases revealed pattern 2, and 2 cases showed pattern 3. In regards to the pattern, 9 cases showed no change (Fig. 2) and 9 cases showed various changes in follow-up ultrasonography (Table 1). Follow-up ultrasound examinations revealed that all 18 cases decreased gradually in size (Table 2). The percentage of decrease was 17.0% in 6 months and 46.7% in 12 months; the decrease was statistically significant (p = 0.011).
In all patients, architectural distortion due to postoperative change was noted by mammography. In four cases, a mass (Fig. 3) was seen at the surgical site. The masses showed oval (n = 3) or irregular (n = 1) shape with spiculated (n = 3) or circumscribed (n = 1) margin. Compared to ultrasonogrphy, there was no tumor recurrence in any of the cases and the size of the masses corresponded well. Focal asymmetry was seen in the remaining seven cases. Calcification was not noted in any of the cases. The mass shadow on mammography decreased gradually in follow-up examinations as the size of lesion decreased as seen on ultrasonography.
Pathologic findings of the two cases that under went either percutaneous needle aspiration or total mastectomy revealed that the isoechoic nodular portion of the lesion corresponded to the dark brownish or cheesy fluid with foreign body reaction, respectively. The case that underwent total mastectomy after mesh implantation showed that periphery of the lesion encapsulated by fibrous tissue and the internal content of the lesion contained amorphous fluid with giant cells and foamy macrophages engulfing proteinaceous materials suggestive of the absorbed mesh itself (Fig. 4).
Absorbable mesh materials have been commonly used in patients who have hernias, renal or splenic rupture, and visceral organ prolapse to seal or reinforce the defected tissue.3-7 Uncommon attempts to use absorbable mesh materials in the field of breast surgery have been presented.2,8,9 Absorbable mesh materials are known to be safe, and have no associated major complications such as infection, hemorrhage, and erosion that are common for nonabsorbable mesh materials.4-7 Polyglactin and polyglycolic acid mesh that are used in the present study are considered to induce minimal tissue reaction, be absorbed completely around 3 months after implantation, and leave a fibrous scar.10-14
The implanted absorbable mesh was supposed to induce reactive fluid or granulation tissue formation and consequently play a role as a volume expander by making a well-encapsulated cystic mass in the site of tissue defect.2 Sanuki et al. analyzed 60 patients who had early-stage breast cancer and underwent breast conserving surgery followed by polyglycolic acid mesh implantation and reported that the images showed dead space lined by granulated scar tissue and filled with fluid.9 In the present study, a well-encapsulated anechoic lesion suggestive of a cystic mass was found in the majority of patients (16/18, 88.9%). Percutaneous needle aspiration of the patient with a pattern 1 lesion revealed that the internal isoechoic nodular portion was fluid content with foreign body reaction. A case of local recurrence of a malignant phyllodes tumor 16 months after surgery showed that the histopathologic findings of cystic mass filled with amorphous fluid and foreign body reaction.
The size of lesions decreased gradually as seen in the follow-up examinations. The percentage of decrease in size reached approximately 47% about 12 months after surgery and it might have influence on the patient's satisfaction.
Mammographic surveillance in patients that have undergone breast conservation due to early-stage breast cancer is an important issue for radiologists as clinical and mammographic surveillance has been demonstrated to be complementary in the detection of local recurrence.15-19 The tumor recurrence rate is estimated to be approximately 1 - 2.5% per year.19 The majority of local tumor recurrences occurred at the lumpectomy site and shared similar imaging and histopathologic findings with the primary tumor. The majority of local recurrence is detected by mammography, in spite of decreased sensitivity of this modality in the surgically treated population.15-21 In the present study, four cases of a mass were noted at the surgical site and supplementary ultrasonographic evaluation was necessary to rule out local recurrence. However, once the author became familiar with ultrasonographic findings of the patients, differentiation between mass made by mesh itself and recurred tumor was not difficult. Goes et al. reported that grouped calcifications were detected in two of 18 patients (11%) 30 months after treatment of breast ptosis with the placement of a mixed (60% polyglactin and 40% polyester) mesh.22 Although calcification of any type was not detected in the present study, newly developed calcification at the surgical site should be evaluated meticulously to rule out cancer recurrence.
The current study has several limitations. First, the number of patients was small. Second, owing to the retrospective nature of the study, follow-up intervals and periods were varied. Third, a longer period of follow-up evaluation might be necessary to assess the feasibility of absorbable mesh implantation after breast partial resection for the purpose of minimizing breast deformity.
In conclusion, the size of the lesion decreased gradually as seen in follow-up examinations. In terms of mammography, architectural distortion due to postoperative change was noted in all patients. In four cases, a mass was seen at the surgical site. However, there was no tumor recurrence in any of the cases. Considering the limitations of mammography on follow-up examinations after breast partial resection and mesh implantation, adjunctive ultrasonography combined with mammography would be recommended in postoperative follow-up examinations.
Figures and Tables
Fig. 1
A sheet of absorbable mesh folded in a fan shape and wrapped with oxidized regenerated cellulose.

Fig. 2
Initial ultrasonography (A) performed about 3 months after surgery of the patient that had undergone wide local excision due to an intraductal papilloma and polyglycolic acid mesh implantation revealed a well-encapsulated anechoic lesion with internal isoechoic nodular portion (pattern 1). The size of the lesion decreased gradually as seen in follow-up examinations about 6 (B) and 12 months after surgery (C).
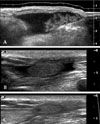
Fig. 3
Postoperative mammogram of the patient, who underwent endoscopic quadrantectomy due to invasive ductal carcinoma, 3 months after surgery revealed a 4 cm sized mass (arrows) at the surgical site. The mass correlated with a well-encapsulated anechoic lesion with internal isoechoic nodular portion (pattern 1) in ultrasonogram (B) performed at the same day.
Fig. 4
A case of total mastectomy 16 months after wide local excision and absorbable mesh implantation due to a recurrent malignant phyllodes tumor showed a radiologic-pathologic correlation. Ultrasonogram (A) and magnetic resonance images (B-D), taken a day before the total mastectomy, showed the recurred tumor mass (arrows) and the well-encapsulated isoechoic lesion made by the implanted absorbable mesh (arrowheads). The implanted mesh showed high signal intensity in T1- (B) and T2- (C) weighted images compared with that of the breast parenchyma and subtle rim enhancement in a subtracted dynamic enhanced T1-weighted image (D). The gross specimen of the resected breast (E) was correlated with the radiologic findings. Microscopic examinations (H & E staining, × 1(F), × 40 (G)) revealed an evacuated cystic lesion (★) encapsulated by fibrous tissue (F) and the internal content containing giant cells and foamy macrophages (G).
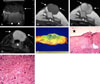
Table 1
Patient Characteristics and Imaging Findings

*Pattern of the lesion (Pattern 1, Pattern 2, Pattern 3).
Size of the lesion (longest × shortest diameter measured in a transverse or a panoramic scan).
†Findings of initial postoperative mammography (interval range from operation and study, 92-350 days; mean, 191 days).
IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ, ADH, atypical ductal hyperplasia; Vicryl, polyglactin mesh; Dexon, polyglycolic acid mesh; PNA, percutaneous needle aspiration.
ACKNOWLEDGMENTS
The author thanks Dr. Won-Gil Bae, who performed breast partial resection and mesh implantation in all study patients.
References
1. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002. 347:1227–1232.

2. Choi Y, Hong HP, Kwag HJ. Ultrasonographic findings of an implanted absorbable mesh in patients with breast partial resection: a preliminary study. J Korean Soc Ultrasound Med. 2007. 26:89–94.
3. Tobias AM, Low DW. The use of a subfascial vicryl mesh buttress to aid in the closure of massive ventral hernias following damage-control laparotomy. Plast Reconstr Surg. 2003. 112:766–776.

4. Dayton MT, Buchele BA, Shirazi SS, Hunt LB. Use of an absorbable mesh to repair contaminated abdominalwall defects. Arch Surg. 1986. 121:954–960.

5. Wainstein MA, Resnick MI. Use of polyglycolic acid mesh to support parenchymal closure following partial nephrectomy. J Urol. 1997. 158:526–527.

6. Rogers FB, Baumgartner NE, Robin AP, Barrett JA. Absorbable mesh splenorrhaphy for severe splenic injuries: functional studies in an animal model and an additional patient series. J Trauma. 1991. 31:200–204.
7. Berry MF, Rosato EF, Williams NN. Dexon mesh splenorrhaphy for intraoperative splenic injuries. Am Surg. 2003. 69:176–180.
8. Goes JC. Periareolar mammaplasty: double skin technique with application of polyglactine or mixed mesh. Plast Reconstr Surg. 1996. 97:959–968.

9. Sanuki J, Fukuma E, Wadamori K, Higa K, Sakamoto N, Tsunoda Y. Volume replacement with polyglycolic acid mesh for correcting breast deformity after endoscopic conservative surgery. Clin Breast Cancer. 2005. 6:175.

10. Boulanger L, Boukerrou M, Lambaudie E, Defossez A, Cosson M. Tissue integration and tolerance to meshes used in gynecologic surgery: an experimental study. Eur J Obstet Gynecol Reprod Biol. 2006. 125:103–108.

11. Krause HG, Galloway SJ, Khoo SK, Lourie R, Goh JT. Biocompatible properties of surgical mesh using an animal model. Aust N Z J Obstet Gynaecol. 2006. 46:42–45.

12. Klinge U, Schumpelick V, Klosterhalfen B. Functional assessment and tissue response of short- and long-term absorbable surgical meshes. Biomaterials. 2001. 22:1415–1424.

13. Barbolt TA. Biology of polypropylene/polyglactin 910 grafts. Int Urogynecol J Pelvic Floor Dysfunct. 2006. 17:Suppl 1. S26–S30.

14. Mounzer AM, McAninch JW, Schmidt RA. Polyglycolic acid mesh in repair of renal injury. Urology. 1986. 28:127–130.

15. Orel SG, Troupin RH, Patterson EA, Fowble BL. Breast cancer recurrence after lumpectomy and irradiation: role of mammography in detection. Radiology. 1992. 183:201–206.

16. Grosse A, Schreer I, Frischbier HJ, Maass H, Loening T, Bahnsen J. Results of breast conserving therapy for early breast cancer and the role of mammographic follow-up. Int J Radiat Oncol Biol Phys. 1997. 38:761–767.

17. Hassell PR, Olivotto IA, Mueller HA, Kingston GW, Basco VE. Early breast cancer: detection of recurrence after conservative surgery and radiation therapy. Radiology. 1990. 176:731–735.

18. Dershaw DD, McCormick B, Osborne MP. Detection of local recurrence after conservative therapy for breast carcinoma. Cancer. 1992. 70:493–496.

19. Giess CS, Keating DM, Osborne MP, Rosenblatt R. Local tumor recurrence following breast-conservation therapy: correlation of histopathologic findings with detection method and mammographic findings. Radiology. 1999. 212:829–835.

20. Liberman L, Van Zee KJ, Dershaw DD, Morris EA, Abramson AF, Samli B. Mammographic features of local recurrence in women who have undergone breast-conserving therapy for ductal carcinoma in situ. Am J Roentgenol. 1997. 168:489–493.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download