Abstract
Monitoring temporal trends of antimicrobial resistance can provide useful information for the empirical selection of antimicrobial agents to treat infected patients and for the control of nosocomial infections. In this study, we analyzed antimicrobial resistance of clinically relevant bacteria in 2003 at Korean hospitals and at a commercial laboratory. The following organism-antimicrobial agent resistance combinations were very prevalent: oxacillin-resistant Staphylococcus aureus (68%), expanded-spectrum cephalosporin-resistant Klebsiella pneumoniae (25%), and fluoroquinolone-resistant Escherichia coli (33%), Acinetobacter spp. (58%), and Pseudomonas aeruginosa (40%). Moreover, gradual increases in vancomycin-resistant Enterococcus faecium (20%), cefoxitin-resistant E. coli (10%) and K. pneumoniae (23%), and imipenem-resistant P. aeruginosa (20%) and Acinetobacter spp. (13%) were also observed. The resistance rates of Acinetobacter spp. to most antimicrobial agents at hospitals and at the commercial laboratory were similar. Among the Acinetobacter spp. isolated at a tertiary-care hospital, 46.2% were multidrug-resistant to 9-12 of 13 antimicrobial agents, and 18.3% were panresistant. The exclusion of duplicate isolates at a tertiary-care hospital significantly lowered the proportion of oxacillin-resistant S. aureus, vancomycin-resistant E. faecium, and fluoroquinolone-resistant E. coli.
Antimicrobial agents have significantly contributed to the improvement of human health and welfare,1 but alarming rises in the prevalence of resistance to some agents among certain groups of bacteria have been noted. The increasing antimicrobial resistance of bacteria is a worldwide problem, albeit the prevalence varies greatly by country because it is influenced by the differences in antimicrobial usage and spread of resistance. With the increase of resistant bacteria, useful information for the treatment of infected patients can be obtained only by determining the susceptibility. However, isolation of etiologic agents is not always possible. Even when possible, it takes time to obtain the results. Therefore, initial antibiotic therapy is typically empirical. Empirical selection of the most appropriate antibiotic is possible only when the current regional resistance pattern is known.
Necessary steps to manage antimicrobial resistance problem include the use of better surveillance to accurately assess the extent of problems. Antimicrobial resistance surveillance is defined as the systematic collection, analysis, and dissemination of data that can be used to identify resistance trends and to assess the need for intervention.2 Monitoring temporal trends of resistance is considered most beneficial for the detection of subtle changes in resistance.3
The most accurate information can be obtained by collecting clinical isolates from participating hospitals and testing them in a reference laboratory. However, this method cannot analyze many isolates and is costly. Examples of such studies include the Alexander, PROTEKT, and SENTRY program.4,5 Another commonly used resistance surveillance method is the analysis of routine susceptibility test data at hospitals because it does not require extensive resources,5,6 although it has inherent inaccuracies due to differences in methodology and interpretation.
Based on a World Health Organization (WHO) recommendation to organize a national surveillance program, the KONSAR study was initiated in 1997.7 In Korea, two surveillance methods have been used: annual analysis of test data generated by KONSAR-participating hospitals,8 and the collection and testing of problematic organism-antimicrobial combinations by the coordinating laboratory.9,10 The latter program showed wide dissemination of metallo-β-lactamase (MBL)-producing Acinetobacter spp. and Pseudomonas spp., and plasmid-mediated AmpC enzyme-producing Escherichia coli and Klebsiella pneumoniae.
In the present surveillance, some modifications were made. Recently, the increase of multidrug-resistant (MDR) Acinetobacter spp. has become a concern in many countries because relatively few antibacterial drugs remain active against this microbe.11 Therefore, in addition to analyzing resistance rates, MDR patterns of Acinetobacter spp. at the coordinating hospital were also analyzed in this study. The higher resistance rates of nosocomially-acquired strains compared to community- acquired strains necessitate differentiation between the two groups. However, in a largescale study it is difficult to separate these groups appropriately. In the previous KONSAR surveillance, data were collected from hospitals only. However, in this study, data were also collected from a commercial laboratory, which examined a large number of specimens submitted mostly from primary-care clinics located outside of Seoul.
In the analysis of resistance data, NCCLS12 recommends excluding duplicate isolates from a single patient, although previous KONSAR surveillances have included duplicate isolates. When duplicate isolates are included, resistance rates increase, particularly in drug-resistant nosocomial pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa. However, the elimination of duplicate isolates may mask trends in emerging resistance.6 In this study, the effect of excluding duplicate isolates on the resistance rates in S. aureus, Enterococcus faecium, and E. coli was analyzed using data from the coordinating hospital.
Routine susceptibility test data on the most clinically relevant aerobic bacteria in 2003 were collected from 44 hospitals located in large cities and small provincial in Korea. The data from five of the 44 hospitals were excluded from analysis due to the hospitals' poor performance in the WHO/CDC quality control program. Less than 10 isolates of non-typhoidal Salmonella and less than 20 isolates of other organisms from a hospital were also excluded from the analysis.
Hospitals were divided into three groups according to location and bed capacity (≥1000 beds countrywide, <1000 beds in Seoul, and <1000 beds in non-Seoul). Resistance rates did not include intermediate susceptibility. The mean resistance rates in each hospital group were calculated from the resistance rates at each hospital, thus minimizing the influence of a large number of isolates and the high prevalence of resistance at some hospitals.5,13 Test data obtained at a commercial laboratory were analyzed separately.
The resistance rates were calculated from all isolates, including duplicate isolates. However, the effect of excluding duplicate isolates was analyzed using the data for S. aureus, E. faecium, and E. coli at the coordinating hospital. MDR patterns of Acinetobacter spp. at the coordinating hospital were analyzed using the WHONET 5 program.14 Statistical analysis of resistance surveillance data, which was considered difficult,2 was not performed in this surveillance, as has been the common practice in large scale and continuous surveillance programs.4,15
The number of isolates in 2003 slightly increased compared to the number in 2002, but the rank order remained identical for 11 of 13 organisms with a large number of isolates (Table 1). The most prevalent species in hospitals was S. aureus (20.0%), while in the commercial laboratory it was E. coli (27.8%). The proportions of E. faecium to E. faecalis differed considerably in hospitals compared to the commercial laboratory, at 81.9% vs. 15.8%, respectively.
To test E. coli, the NCCLS disk diffusion method, broth microdilution method (Vitek [bioMerieux, Marcy l'Etoile, France] or MicroScan [Dade MicroScan Inc., West Sacramento, CA, U.S.A.] system), or a combination of the two methods were used by 11, 27, and three laboratories, respectively. Oxacillin disks were used to differentiate oxacillin (methicillin)-resistant staphylococci and to screen penicillin G-non-susceptible pneumococci. To test fluoroquinolone susceptibility of gram-negative bacilli, the majority of hospitals used ciprofloxacin, while others used levofloxacin.
The antimicrobial agents used in 2003 to test the susceptibility of E. coli and S. aureus were similar to those used in 2002 (Table 2). Less than 70% of the hospitals tested the susceptibility of E. coli to cephalothin, piperacillin, and cotrimoxazole, and only 50% of the hospitals tested the susceptibility of S. aureus to cotrimoxazole. Method analysis of 38 laboratories showed that the types of antimicrobial agents tested were 8-10, 15-20, and 16-21, at 5, 20, and 13 laboratories, respectively (data not shown).
The mean resistance rates (Table 3) of S. aureus were much higher than those of coagulase-negative staphylococci (CNS) to clindamycin (59% vs. 36%) and to ciprofloxacin (61% vs. 38%), but slightly lower to oxacillin (68% vs. 73%) and to cotrimoxazole (16% vs. 39%). Seventy percent of Streptococcus pneumoniae were resistant to oxacillin, suggesting penicillin non-susceptibility. The ampicillin and vancomycin resistance rates of E. faecium were 88% and 20%, respectively.
The cefotaxime and fluoroquinolone resistance rates of E. coli were 11% and 33%, respectively, and those of K. pneumoniae to ceftazidime and cefoxitin were 25% and 23%, respectively (Table 4). The lowest resistance rates shown by Enterobacter cloacae and S. marcescens to cephalosporins were to cefepime and were 8% and 19%, respectively. Imipenem-resistant K. pneumoniae, E. cloacae, and Serratia marcescens isolates existed, although their rates were very low.
Lower resistance rates were shown by P. aeruginosa to ceftazidime (19%) and imipenem (20%) and by Acinetobacter spp. to imipenem (13%) and cefoperazone-sulbactam (15%). The resistance rates of non-typhoidal Salmonella to ampicillin, cotrimoxazole, and fluoroquinolone were 29%, 4%, and 0.3%, respectively (data not shown). The ampicillin resistance rate of Haemophilus influenzae was 54%, and a test of part of the isolates showed that 52% produced β-lactamase (data not shown).
The resistance rates of staphylococci to all antimicrobial agents, except for cotrimoxazole resistance of S. aureus (Table 3), were much lower at the commercial laboratory than at the hospitals. The ampicillin resistance rates of E. faecium were equal at the hospitals and the commercial laboratory (88%), but the vancomycin resistance rates were 20% at the hospitals and 7% at the commercial laboratory. Compared to the resistance rates at the hospitals, the rates at the commercial laboratory were: similar in cefotaxime and fluoroquinolone resistance of E. coli (12% and 31%, respectively); slightly lower in cefoxitin resistance of K. pneumoniae (18%); much lower in cefotaxime, ticarcillin-clavulanic acid, amikacin, and fluoroquinolone resistance of E. cloacae and S. marcescens; slightly lower in ceftazidime and imipenem resistance of P. aeruginosa (15% and 17%, respectively); and much lower in ampicillin-sulbactam and imipenem resistance of Acinetobacter spp. (22% and 5%, respectively) (Table 4).
The trends of significant resistance in staphylococci, enterococci, K. pneumoniae, and Acinetobacter spp. are shown in Fig. 1-3. As in 2002, the oxacillin resistance rate was slightly higher in CNS than in S. aureus in 2003 (Fig. 1). Not only did the ampicillin resistance of E. faecium remain highly prevalent, but vancomycin resistance reached 20% in 2003. The ceftazidime and cefoxitin resistance rates of K. pneumoniae in 2003 were higher than those in 2002, and a gradual increase in the fluoroquinolone and amikacin resistance rates is apparent in the last several years (Fig. 2). A slight decline of Acinetobacter spp. resistance to amikacin, fluoroquinolone, and ceftazidime was noted, but the rates remained over 50% (Fig. 3). Imipenem-resistant Acinetobacter spp. gradually increased, reaching 13% in 2003.
Comparing the resistance rates between hospital groups, vancomycin-resistant E. faecium, fluoroquinolone-resistant E. coli, and cefoxitin-resistant K. pneumoniae were more prevalent at larger hospitals. Large differences in resistance rates were observed between the large hospital group and the commercial laboratory for vancomycin-resistant E. faecium (25% and 7%, respectively), and for imipenem-resistant Acinetobacter spp. (11% and 5%, respectively) (Fig. 4).
MDR patterns of Acinetobacter spp. to 13 antimicrobial agents were determined excluding duplicate isolates and isolates with less than a 1% pattern (Fig. 5). Among the 925 isolates analyzed, 11.5% were resistant to none of the antimicrobial agents (data not shown), but 18.3% were resistant to all of the drugs, and 11.5%, 11.6% and 18.6% showed MDR to 10, 11, and 12 antimicrobial agents, respectively.
Resistance rates were lowered by more than 20% after the exclusion of duplicate isolates from the coordinating laboratory data of the following: clindamycin and cotrimoxazole resistance of S. aureus, teicoplanin and vancomycin resistance of E. faecium, and ampicillin-sulbactam, ticarcillin-clavulanate, piperacillin-tazobactam, cefotaxime, ceftazidime, aztreonam, cefoperazone-sulbactam, cefoxitin, cefotetan, amikacin tobramycin, and ciprofloxacin resistance of E. coli (Table 5).
It is stressed that surveillance is an important part of modern clinical microbiology, because only resistance surveillance can provide the necessary information for empirical selection. For example, it has been suggested that when resistance to a particular drug occurs in >10-20% of isolates, the drug should not be used for empirical treatment.6 Isolation rank based on the first isolate in a patient has been recommended by NCCLS12 for reporting laboratory surveillance data, with the primary aim of guiding clinicians in the selection of empirical therapy. However, when only the first isolates are included, resistance selection occurring within the observation period cannot be detected.6 In our present study, duplicate isolates were included, as in the previous surveillances, because we considered the information gained by including duplicate isolates to be useful for the control of nosocomial infections.
The proportions of each species were very similar in 2002 and 2003: S. aureus, E. coli, P. aeruginosa, CNS, and K. pneumoniae remained in the same rank order of 1 to 5, respectively. However, at the commercial laboratory, E. coli, P. aeruginosa, and S. aureus were ranked 1 to 3, respectively. It is difficult to compare the rank orders without knowledge of the specimens, but it can be assumed that the difference was probably due to a relatively low prevalence of nosocomial pathogens at clinics (Table 1).
With the increase in MDR bacteria, limiting susceptibility testing only to a few classes of antimicrobial agents cannot provide sufficient information for patient treatment. However, as in 2002, some laboratories tested susceptibility to only a small number of antimicrobial agents (Table 2). For optimal detection of extended-spectrum β-lactamase (ESBL)-producing E. coli and K. pneumoniae, the use of both ceftazidime and cefotaxime is recommended.16 Due to the increasing prevalence of CTX-M type ESBLs, the concomitant testing of cefotaxime susceptibility is now important in Korea,17 as in other countries.18 However, some laboratories tested susceptibility to only one expanded-spectrum cephalosporin, as was the case in 2002. A previous study with collected strains showed a high prevalence of plasmidmediated AmpC enzyme DHA-1-producing K. pneumoniae,10 indicating the need for susceptibility testing to cefoxitin and cefepime. However, many laboratories did not test for these drugs in 2002 or 2003.
The present surveillance showed that the oxacillin-resistant S. aureus (ORSA) and oxacillin-resistant CNS remained very high, at approximately 70% (Fig. 1). This trend is similar to that in Japan.19 ORSA is now a worldwide problem: the proportions were more than 40% in Greece, Italy, and the United Kingdom in 1999-2002.20 In the present surveillance, penicillin-non-susceptible pneumococci (based on the breakpoint for the treatment of meningitis) also remained very prevalent as was observed in other Asian countries.21 Increases in penicillin resistance were also reported in the United States in the 1990s.22
The gradual increase of vancomycin-resistant E. faecium continued and reached 20% in the hospital groups in 2003 (Fig. 2). The rate was much higher in the large hospital group (25%) than in the smaller non-Seoul hospital group (14%), and was only 7% in the commercial laboratory (Table 4), suggesting the influence of nosocomial dissemination. The higher rate observed at a tertiarycare hospital was partly due to the inclusion of duplicate isolates (Table 5). Difficulties in the control of vancomycin-resistant enterococci were shown by their high prevalence in a U.S. hospital.23 E. faecium increased from 12.7% to 22.2% from 1993 to 2002 among all enterococcal isolates, which included one isolate per patient per month, and vancomycin-resistant isolates increased from 28.9% to 72.4%, respectively.
The high prevalence of ampicillin-resistant E. coli (71%) in our study was similar to the rate in Taiwan (78%).24 The ampicillin resistance rate of H. influenzae (53%) was similar to the β-lactamase positive rate of 52% (data not shown). This result suggests that β-lactamase-negative ampicillin-resistant (BNAR) H. influenzae remains rare in Korea, although it is a prevalent type in Japan.25
The increase in isolates of ampicillin-resistant non-typhoidal Salmonella may indicate an increasing prevalence of this resistance in the community, as the infections are more often community-acquired rather than nosocomially-acquired. However, the resistance rates were much lower than those of Salmonella enterica serovar Typhymurium DT104 isolates, which were 34% for both ampicillin and cotrimoxazole.26 The ciprofloxacin resistance rate was only 0.3%, but this rate probably cannot be used to predict clinical efficacy in the treatment of extraintestinal infections because low-level quinolone resistance cannot be detected by using fluoroquinolones.16
E. coli and K. pneumoniae often acquire ESBL genes. In this study, 11% of E. coli and 25% of K. pneumoniae were resistant to cefotaxime and ceftazidime, respectively, suggesting ESBL production. In Korea, TEM-, SHV-, and CTX-M-type ESBLs were previously reported.17,27 Cefoxitin-resistant E. coli (10%) and K. pneumoniae (23%) may be partly due to plasmid-mediated AmpC enzymes, such as DHA-1 and CMY-1-like, which are prevalent in Korea.10 Carbapenems are the only class of β-lactam agents active against strains producing ESBLs and hyperproducing AmpC enzymes.28 Therefore, the increasing carbapenem resistance in P. aeruginosa and Acinetobacter spp. is the most serious problem. The imipenem resistance rate of Acinetobacter spp. (13%) was lower than that of P. aeruginosa (20%), but interestingly, the rate of the former has increased rapidly since 2002 (Fig. 3), while that of the latter remained similar (data not shown). Among the isolates in 2000 and 2001, VIM-2 and IMP-1 MBL genes were detected in Acinetobacter isolates, but only the VIM-2 gene was detected in P. aeruginosa isolates.9 Kim et al.29 tested 116 imipenem-resistant P. aeruginosa isolates from 2000 to 2003 at a tertiary-care hospital, and detected 19 (16.4%) VIM-2 gene-positive isolates. These data indicate a wide dissemination of MBL-producing P. aeruginosa and Acinetobacter spp. A study with 3,233 strains of P. aeruginosa isolated at 37 medical institutes in Japan showed an imipenem resistance rate of 19.0%, which was similar to our results, but the rates to all other antimicrobial agents were lower than our results.30 Jeong et al.31 reported a VIM-2-producing E. cloacae isolate in 2003. In the present study, imipenem-resistant isolates were also present in K. pneumoniae, although the proportion was very low.
The resistance of E. coli and K. pneumoniae to gentamicin and tobramycin in this study were not very high (21% and 32%, respectively). Previously, amikacin-resistant gram-negative bacilli were very rare. However, the resistance rates of gram-negative bacilli other than E. coli were high at 10% to 54% (Table 4), making empirical use of the drug difficult. Fluoroquinolones are frequently used, as they are one of the three major broad-spectrum classes of antimicrobial agents.32 The fluoroquinolone resistance rate of E. coli in 2003 (33%) was similar to that in 2002. We found similar fluoroquinolone resistance rates of E. coli, P. aeruginosa and Acinetobacter spp. at the commercial laboratory and at the hospitals (Table 4), which also suggests the prevalence of this resistance in the community.
It is concerning that Acinetobacter spp. isolates are often MDR. Abbo et al.33 reported that they were unable to clearly define the mode of spreading and the reason for the emergence of MDR A. baumannii. Analysis of Acinetobacter spp. isolated at the coordinating laboratory showed that 46.2% of the isolates were MDR to 9 to 12 of the 13 antimicrobial agents tested, and 18.3% were panresistant (Fig. 5). MDR and panresistant Acinetobacter has been reported in other countries as well.33,34 In Brazilian hospitals, resistance rates of Acinetobacter spp. to carbapenem have reached 12% or higher.35 Thus, more toxic agents, such as polymyxin, have been used. Consequently, it was found that 5 of 100 Acinetobacter blood isolates were resistant to this drug.
Ideally, multi-center surveillance should be representative of all types and sizes of institutions; however, current surveillance tends to include only larger university-affiliated hospitals, which may overrepresent the prevalence of resistance due to the types of patients treated at large hospitals.36 Accurate resistance surveillance of a community-acquired pathogen was considered to be difficult because of sampling bias. For example, physicians' requests for urine culture have decreased recently, and cultures have been performed relatively more often for patients with failed antimicrobial therapy.36 In the analysis of surveillance data, making the distinction between nosocomial and community-acquired infections is also difficult.15 In the present study, as in previous studies, we did not separate community-acquired from nosocomially-acquired strains due to the difficulty of separation. Instead, the resistances of strains at a commercial laboratory, which were mostly from primary-care clinic patients, were compared to those at hospitals. Our results showed that resistance rates at the commercial laboratory were generally lower, but it was difficult to generalize the resistance patterns.
It is well known that excluding duplicate isolates lowers resistance rates. Lee et al.37 reported an overestimation of MRSA when duplicate isolates were included. In our analysis of data from a tertiary-care hospital, including only the first isolate/patient/year reduced proportions of ORSA by 18%, vancomycin-resistant E. faecium by 28%, and fluoroquinolone-resistant E. coli by 24% (Table 5). However, the effect was minimal in the resistance rates to old antimicrobial agents, i.e., penicillin-resistant S. aureus, ampicillin-resistant E. faecium, and ampicillin- and fluoroquinolone-resistant E. coli. When duplicate isolates are excluded from analysis, the emergence of resistance that occurs within the observation period may not be seen, thereby giving an overly optimistic view of the percentage of susceptible strains.3,6 Also, there could be a question as to the removal period, i.e., 5 days, 30 days, or 365 days.
In conclusion, oxacillin-resistant staphylococci, expanded-spectrum cephalosporin-resistant K. pneumoniae, and fluoroquinolone-resistant E. coli, Acinetobacter spp., and P. aeruginosa remain very prevalent in Korea. Vancomycin-resistant E. faecium, cefoxitin-resistant E. coli and K. pneumoniae, and imipenem-resistant P. aeruginosa and Acinetobacter spp. have gradually increased. The resistance rates of Acinetobacter spp. to most antimicrobial agents tested at hospitals and at a commercial laboratory were similar, suggesting dissemination of this problematic organism in Korea. Analysis of the data from a tertiary-care hospital showed the prevalence of MDR and panresistant Acinetobacter spp. The exclusion of duplicate isolates lowered the proportion of ORSA, vancomycin-resistant E. faecium, and fluoroquinolone-resistant E. coli significantly. In future surveillances, more useful information could be obtained by determining the resistance trends of problematic antibiotic-bacteria combinations at both hospitals and commercial laboratories. Also, more information could be gained by presenting resistance data that both includes and excludes duplicate isolates.
Jae Seok Kim, Hallym University College of Medicine, Seoul; Sunjoo Kim, Gyeongsang National University Hospital, Jinju; Namhee Ryoo, Dong San Medical Center, Keimyong University, Taegu; Mun-Yeun Kim, Dongkook University Pohang Hospital, Pohang; Chulhun L. Chang, College of Medicine, Pusan National University, Busan; Mi-Na Kim, Ulsan University Asan Medical Center, Seoul; Wee-Gyo Lee, Ajou University Hospital, Suwon; Myungshin Kim, Catholic University of Korea, St. Mary's Hospital, Seoul; Chea-Hoon Lee, Yeung-Nam University Hospital, Taegu; Jeong Ho Kim, Yongdong Severance Hospital, Seoul; Joseph Jeong, Ulsan University Hospital, Ulsan; Ji Hyun Cho, Wonkwang University Hospital, Iksan; Young Uh, Yonsei University Wonju Christian Hospital, Wonju; Ki Sook Hong, Ewha Womans University Tongdaemun Hospital, Seoul; Bo Moon Shin, Sanggye Paik Hospital, Inje University College of Medicine, Seoul; Hye-Soo Lee, Chonbuk National University Medical College, Chonju; Sook Jin Jang, Chosun University Hospital, Kwangju; Ae Ja Park, Chung Ang University Pil-dong Hospital, Seoul; Young Joo Cha, Chung Ang University Yong San Hospital, Seoul; Young Jin Choi, Soonchunhyang Chunan Hospital, Chunan; Sung Ha Kang, Hallym University School of Medicine, Chunchon Sacred Heart Hospital, Chunchon; Chang Hyun Rhim, Wallace Memorial Baptist Hospital, Busan; Myung Hee Lee, Korea Veterans Hospital, Seoul; Wonkeun Song, Hallym University College of Medicine, Seoul; Tae Yeal Choi, College of Medicine, Hanyang University, Seoul; Eui-Chong Kim, Seoul National University College of Medicine, Seoul; Jung Oak Kang, College of Medicine, Hanyang University, Kuri; Yeon Joon Park, College of Medicine, Catholic University of Korea, Seoul; Seong Geun Hong, College of Medicine, Pochon CHA University, Seongnam; Young Ah Kim, National Health insurance Corporation Ilsan Hospital, Goyang; Hee Joo Lee, Kyung Hee University Hospital, Seoul; Jongwook Lee, Konyang University Hospital, Daejeon; Young-Joon Lee, National Cancer Center, Kyunggi; Miae Lee, Ewha Womans University Mokdong Hospital, Seoul; Hee-Bong Shin, Soonchunhyang University Hospital, Bucheon; Young Ree Kim, Cheju National University Hospital, Cheju; Seung-Ok Lee, Seoul Clinical Laboratories, Seoul; Sung-Hee Lee, Cheju Hanmaeum Hospital, Cheju, Korea.
Figures and Tables
Fig. 1
The resistance trends of staphylococci to oxacillin, and E. faecium to ampicillin and vancomycin. Continued high prevalence of oxacillin-resistant staphylococci, ampicillin-resistant E. faecium, and a gradual increase of vancomycin-resistant E. faecium were observed. OXA, oxacillin; AMP, ampicillin; VAN, vancomycin; R, resistant; SAU, S. aureus; CNS, coagulase-negative staphylococci; EFM, E. faecium.

Fig. 2
The resistance trend of K. pneumoniae to cefoxitin, ceftazidime, amikacin, and fluoroquinolone. The ceftazidime resistance rate remained high, while a tendency of increasing resistance to other antimicrobial agents was observed. FOX, cefoxitin; CAZ, ceftazidime; AMK, amikacin; FQN, fluoroquinolone.

Fig. 3
The resistance trend of Acinetobacter spp. to amikacin, fluoroquinolone, and ceftazidime remained high, and a tendency for increasing imipenem-resistance was observed. AMK, amikacin; FQN, fluoroquinolone; CAZ, ceftazidime; IMP, imipenem.

Fig. 4
Antimicrobial resistances of strains isolated at three hospital groups and tested at a commercial laboratory. Resistance rates were generally higher in the large hospital group. Vancomycin-resistant E. faecium and imipenem-resistant Acinetobacter spp. were much less prevalent among the commercial laboratory tested strains. S-med, Seoul-medium; N-med, non-Seoul-medium; Comm Lab, commercial laboratory; VAN, vancomycin; FQN, fluoroquinolone; FOX, cefoxitin; IPM, imipenem; EFM, E. faecium; ECO, E. coli; KPN, K. pneumoniae; PAE, P. aeruginosa; ABA, A. baumannii.

Fig. 5
Multi-resistance of Acinetobacter spp. isolated at a tertiary-care hospital. Among the isolates 11.5% were resistant to none of the 13 antimicrobial agents tested, but 18.3% were resistant to all of the agents tested.
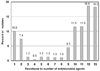
Table 2
Proportion of Hospitals Which Used Antimicrobial Agents for the Susceptibility Testing of E. coli and S. aureus in 2002 and 2003*

Table 3
Antimicrobial Resistance Rates of Staphylococci, Pneumococci, and Enterococci Tested at Hospitals and at a Commercial Laboratory

References
1. Wise R. The relentless rise of resistance? J Antimicrob Chemother. 2004. 54:306–310.
2. Bax R, Bywater R, Cornaglia G, Goosens H, Hunter P, Isham V, et al. Surveillance of antimicrobial resistancewhat, how and whither? Clin Microbiol Infect. 2001. 7:316–325.
3. Morris AK, Masterton RG. Antibiotic resistance surveillance: action for international studies. J Antimicrob Chemother. 2002. 49:7–10.
4. Felmingham D, Grueneberg RN. Alexander Project group. The Alexander project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000. 45:191–203.
5. Van Beneden CA, Lexau C, Baughman W, Barnes B, Bennett N, Cassidy PM, et al. Aggregated antibiograms and monitoring of drug-resistant Streptococcus pneumoniae. Emerg Infect Dis. 2003. 9:1089–1095.
6. Cornaglia G, Hryniewicz W, Jarlier V, Kahlmeter G, Mittermayer H, Stratchounski L, et al. European recommendations for antimicrobial resistance surveillance. Clin Microbiol Infect. 2004. 10:349–383.
7. Chong Y, Lee K, Park YJ, Jeon DS, Lee MH, Kim MY, et al. Korean nationwide surveillance of antimicrobial resistance of bacteria in 1997. Yonsei Med J. 1998. 39:569–577.
8. Lee K, Kim YA, Park YJ, Lee HS, Kim MY, Kim E-C, et al. Increasing prevalence of vancomyin-resistant enterococci, and cefoxitin-, imipenem- and fluoroquinolone-resistant gram-negative bacilli; a KONSAR study in 2002. Yonsei Med J. 2004. 45:598–608.
9. Lee K, Lee WG, Uh Y, Ha GY, Cho J, Chong Y, et al. VIM- and IMP-type metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg Infect Dis. 2003. 9:868–871.
10. Lee K, Lee M, Shin JH, Lee MH, Kang SH, Park AJ, et al. Plasmid mediated ampC genes in cefoxitin-resistant E. coli and K. pneumoniae clinical isolates. 2004. In : 44th Interscience Conference of Antimicrobial Agents and Chemotherapy; Washington, DC.. Abstr. C2-1339.
11. Urban C, Segal-Maurer S, Rahal JJ. Considerations in control and treatment of nosocomial infections due to multidrug-resistant Acinetobacter baumannii. Clin Infect Dis. 2003. 36:1268–1274.
12. National Committee for Clinical Laboratory Standards. Analysis and presentation of cumulative antimicrobial susceptibility test data. Approved guideline M39-A. 2002. Wayne, PA: NCCLS.
13. Fridkin SK, Hill HA, Volova NV, Edwards JR, Lawton RM, Gaynes RP, et al. Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project Hospitals. Temporal changes in prevalence of antimicrobial resistance in 23 U.S. hospitals. Emerg Infect Dis. 2002. 8:697–701.
14. Stelling JM, O'Brien TF. Surveillance of antimicrobial resistance: the WHONET program. Clin Infect Dis. 1997. 24:S157–S168.
15. Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database-USA. Clin Infect Dis. 1999. 29:259–263.
16. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: thirteen's informational supplement. 2003. Wayne, PA: NCCLS.
17. Pai H, Choi E-W, Lee H-J, Hong JY, Jacoby GA. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol. 2001. 39:3747–3749.
18. Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004. 48:1–14.
19. Yasunaka K, Kono K. Epidemiological study of methicillin-resistant Staphylococcus aureus at Fukuoka University Hospital. Microb Drug Resist. 1999. 5:207–213.
20. Tiemersma EW, Bronzwaer SLAM, Lyytikainen O, Degener JE, Schrijnemakers P, Bruinsma N, et al. Methicillin-resistant Staphylococcus aureus in Europe, 1999-2002. Emerg Infect Dis. 2004. 10:1627–1634.
21. Song J-H, Jung S-I, Ki HK, Shin M-H, Ko KS, Son JS, et al. Clinical outcomes of pneumococcal pneumonia caused by antibiotic-resistant strains in Asian countries: a study by the Asian Network for Surveillance of Resistant Pathogens. Clin Infect Dis. 2004. 38:1570–1578.
22. Mera RM, Miller LA, Daniels JJD, Weil JG, White AR. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States over a 10-year period: Alexander Project. Diag Microbiol Infect Dis. 2005. 51:195–200.
23. Treitman AN, Yarnold PR, Warren J, Noskin GA. Emerging incidence of Enterococcus faecium among hospital isolates (1993 to 2002). J Clin Microbiol. 2005. 43:462–463.
24. Lauderdale TL, McDonald LC, Shiau YR, Chen PC, Wang HY, Lai JF, et al. The status of antimicrobial resistance in Taiwan among gram-negative pathogens: the Taiwan surveillance of antimicrobial resistance (TSAR) program, 2000. Diag Microbiol Infect Dis. 2004. 48:211–219.
25. Hasegawa K, Chiba N, Kobayashi R, Murayama Y, Iwata S, Sunakawa K, et al. Rapidly increasing prevalence of β-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob Agents Chemother. 2004. 48:1509–1514.
26. Park M-S, Kang Y-H, Lee S-J, Song C-Y, Lee B-K. Characteristics of epidemic multidrug-resistant Salmonella enterica serovar Typhimurium DT104 strains first isolated in Korea. Korean J Infect Dis. 2002. 34:1–8.
27. Jeong SH, Bae IK, Lee JH, Sohn SG, Kang GH, Jeon GJ, et al. Molecular characterization of extended-spectrum beta-lactamases produced by clinical isolates of Klebsiella pneumoniae and Escherichia coli from a Korean Nationwide Survey. J Clin Microbiol. 2004. 42:2902–2906.
28. Livermore DM, Woodford N. Carbapenemases: a problem in waiting? Curr Opin Microbiol. 2000. 3:489–495.
29. Kim IS, Oh WI, Song JH, Lee NY. Screening and identification of metallo-β-lactamase gene in clinical isolates of imipenem-resistant Pseudomonas aeruginosa. Korean J Lab Med. 2004. 24:177–182.
30. Tsuji A, Kobayashi I, Oguri T, Inoue M, Yabuuchi E, Goto S. An epidemiological study of the susceptibility and frequency of multiple-drug-resistant strains of Pseudomonas aeruginosa isolated at medical institutes nationwide in Japan. J Infect Chemother. 2005. 11:64–70.
31. Jeong SH, Lee K, Chong Y, Yum JH, Lee SH, Choi HJ, et al. Characterization of a new integron containing VIM-2, a metallo-β-lactamase gene cassette, in a clinical isolate of Enterobacter cloacae. J Antimicrob Chemother. 2003. 51:397–400.
32. Hooper DC. The future of the quinolones. APUA Newsletter. 2001. 19:1–5.
33. Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y. Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2005. 11:22–29.
34. Kuo LC, Teng LJ, Yu CJ, Ho SW, Hsueh PR. Dissemination of a clone of unusual phenotype of pandrug-resistant Acinetobacter baumannii at a university hospital in Taiwan. J Clin Microbiol. 2004. 42:1759–1763.
35. Reis AO, Luz DAM, Tognim MCB, Sader HS, Gales AC. Polymyxin-resistant Acinetobacter spp. isolates; what is next? Emerg Infect Dis. 2003. 9:1025–1027.
36. Critchley IA, Karlousky JA. Optimal use of antibiotic resistance surveillance systems. Clin Microbiol Infect. 2004. 10:502–511.
37. Lee S-O, Cho YK, Kim S-Y, Lee ES, Park SY, Seo Y-H. Comparison of trends of resistance rates over 3 years calculated from results for all isolates and for the first isolate of a given species from a patient. J Clin Microbiol. 2004. 42:4776–4779.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download