Abstract
Hyperlipidemia is a rare cause of pancreatitis. It has been believed that free fatty acids released from hydrolyzed serum chylomicrons or triglycerides and chylomicrons induce hyperlipidemic pancreatitis by damaging acinar cells and capillaries. Type I, IV or V hyperlipidemic (Fredrickson's classification) pancreatitides have distinctive features of increased and heightened serum chylomicron and triglyceride levels. In contrast, type IIb hyperlipidemia usually doesn't have increased chylomicrons. It is a dominant inherited genetic disorder and doesn't manifest the subjective symptom before combining vascular complications such as coronary artery disease. Only a few cases of type IIb hyperlipidemic pancreatitis have been reported. We experienced a male patient with recurrent hyperlipidemic pancreatitis combined with type IIb hyperlipidemia. We present the case report and a review of the literature of hyperlipidemic pancreatitis, especially cases in Korea.
Hypertriglyceridemia is a rare cause of pancreatitis.1 As known, serum pancreatic enzymes may be normal or minimally elevated, even in the presence of severe pancreatitis diagnosed by imaging studies. A serum triglyceride level of more than 1,000 to 2,000 mg/dL in a patient with type I, IV, V hyperlipidemia (Fredrickson's classification) is an identifiable risk factor of acute pancreatitis.2,3
The typical profile of hyperlipidemic pancreatitis is a preexisting lipid abnormality along with secondary factor such as poorly controlled diabetes or alcohol abuse. Less commonly, a patient with isolated hyperlipidemia (types V and I) without precipitating factors also presents with pancreatitis. Familial combined hyperlipidemia has also been described as a disorder in which multiple lipoprotein phenotypes (types IIa, IIb, and IV) occur in the same family.4-6
Hyperlipidemic pancreatitis associated with type I, IV or V hyperlipoproteinemia of Fredrickson's classification has been reported, but not type IIb hyperlipoproteinemia with associated pancreatitis. Recently, we experienced a patient with hyperlipidemic pancreatitis due to type IIb hyperlipoproteinemia. So, we present a patient who had recurrent pancreatitis associated with type IIb hyperlipoproteinemia and review the literature in Korea.
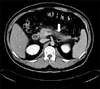
A 28-year-old obese man presented at the Emergency Room of our hospital complaining of epigastric pain radiated to the back for one day. He had been admitted to our hospital 3 times previously due to acute pancreatitis with hyperlipidemia. He had type 2 diabetes mellitus and active pulmonary tuberculosis. He was treated with dietary restriction, daily NPH insulin injection, and lipid lowing medication such as fibrate derivatives. His father was noted to have hypertension, old cerebral infarction and type 2 diabetes mellitus. His mother had hyperlipidemia (type IV) and his elder brother had recurrent acute pancreatitis and diabetes mellitus. He didn't have xanthoma or xanthelasma. He had no alcohol drinking history and no biliary calculus disease. His height was 176 cm and weight was 87 kg, showing marked obesity. On physical examination, his abdomen was slightly distended with hypoactive bowel sounds and he had direct tenderness on epigastrium without rebound tenderness. On admission, laboratory data showed white blood cell count of 15,210/mm3. Blood chemistry tests revealed fasting blood sugar level of 228 mg/dL, lactate dehydrogenase level of 379 IU/L, and aspartate aminotransferase level of 20 IU/L. The serum amylase level of 160 mg/dL was slightly higher than the normal range (25~125 mg/dL). Serum lipase level was increased to 1596 U/L (normal range of 13~60 U/L). Serum calcium level and blood urea nitrogen level were within normal limits. The lipid profiles such as total cholesterol, triglyceride, high density cholesterol and low density cholesterol were 272 mg/dL, 605 mg/dL, 28 mg/dL and 98 mg/dL, respectively. A diagnosis of type IIb hyperlipoproteinemia was made based on serum lipoprotein electrophoresis [0% of chylomicron (0-2.0), 62.9% of β-lipoprotein (36.5-59.1), 22.5% of pre-β-lipoprotein (3.8-33.8), and 14.6% of α-lipoprotein (20.4-46.4)]. Hemoglobin A1c level was 10.4% and fasting C-peptide and postprandial C-peptide were 2.7 and 3.7 ng/mL, respectively. Abdominal CT scan revealed diffusely enlarged pancreas, peripancreatic fat infiltration and pelvic ascites, which were compatible with acute pancreatitis grade C (Fig. 1). He was treated with dietary restriction, hydration, NPH insulin injection and lipid lowering agents. For the purpose of distinguishing infective pancreatitis from hyperlipidemic pancreatitis, we prescribed antibiotics (2 g of meropenem® per day) for two days until abdominal CT information was obtained. His abdominal pain was resolved within a few days, and white blood cell count was normalized. He was discharged after 8 days, with complete recovery from acute pancreatitis.
Since Speck in 1865 reported the association between hyperlipidemia and acute pancreatitis, the relationship has been discussed in many manuscripts.1,3,7-10 Havel et al suggested a mechanism that hypertriglyceridemia leads to pancreatitis.11 The mechanism is thought to be that serum chylomicrons or triglycerides are hydrolyzed by lipase in the pancreatic capillaries to release free fatty acids. Free fatty acids bind Ca2+ and produce pancreatic capillary damage or microthrombi, leading to pancreatitis. Unbound free fatty acids have a toxic effect on acinar cells or may injure capillary vessels. Increased concentration of chylomicrons in the pancreatic capillaries causes capillary plugging and leads to ischemia and acidosis, and in the acidic environment, free fatty acids activate trypsinogen and initiate acute pancreatitis.11
It has been believed that two signs of hyperlipidemic pancreatitis are high serum triglyceride level above 1,000 mg/dL and the appearance of chylomicron in the serum at the early stage of pancreatitis.3 Thus, lipoprotein electrophoresis of hyperlipidemic pancreatitis is exhibited most commonly with type V hyperlipidemia, followed by types I, and IV hyperlipidemia.2,8 It is changed to type I, IV or III hyperlipidemia in the recovery stage of acute pancreatitis.12
In our case, the patient showed type IIb hyperlipidemia. There were several mismatches compared with typical hyperlipidemic pancreatitis in our case. Firstly, the triglyceride level was only 605 mg/dL which was not compatible with the belief that hyperlipidemic pancreatitis is associated with a serum triglyceride level of more than 1,000 mg/dL. According to Ohmoto et al, there were two cases of pancreatitis associated with a serum triglyceride level of lower than 1,000 mg/dL, among 33 cases of hyperlipidemic pancreatitis in Japan.13 Cho et al. reported hyperlipidemic pancreatitis with a serum triglyceride level of 416 mg/dL in Korea.14 Secondly, there was no chylomicron in type IIb hyperlipidemia, so the role of chylomicron in hyperlipidemic pancreatitis was not explained. A few case reports have shown that lipid profile was changed during the recovery period of pancreatitis.12,13,15-17 One possible explanation is that our patient visited in a fasting state one day after the onset of the disease because he had already been known to relieve the subjective symptom by fasting learned through his previous experiences of pancreatitis. This may have lowered the serum triglyceride and chylomicron levels rapidly.
Our patient had suffered pancreatitis on 3 occasions. Unfortunately, serum lipoprotein electrophoresis was not been done, and therefore Fredrickson's classification was not made. However, on all 3 admissions, serum levels of triglyceride were lower than 1000 mg/dL: 687 mg/dL, 927 mg/dL, and 451 mg/dL. During the follow up period, his serum triglyceride levels were frequently recorded at more than 1000 mg/dL without pancreatitis symptoms. Serum hemoglobin A1c level ranged from 7.7 to 11.0%, despite using lipid lowering agents, such as gemfibrozils and fibrate derivatives, and insulin therapy. Huang and Raskin suggested that anti-hyperlipidemic medications alone are usually not sufficient to control hyperlipidemia in diabetic patients with hypertriglyceridemia, and that antidiabetic medications must be used.18 Poorly controlled diabetes may induce hyperlipidemic pancreatitis in the patient with type IIb hyperlipidemia. However, the mechanism remains unclear, further research is indicated.
In Korea, hyperlipidemic pancreatitis was first reported by Lee et al. in 1986, after which six cases, including our own, have been reported (Table 1).14-17,19,20 Most cases tended to have mild to moderate symptoms and to be repeated episodes of pancreatitis, except two cases, one with pregnancy and the other with acromegaly. Five cases had diabetes mellitus. The most common patient age was the third and fourth decade, ranging from 25 to 45 years (average: 33.1 years). Only two patients had levels of serum triglyceride lower than 1000 mg/dL, and one had more than 2000 mg/dL. Three cases were type V hyperlipidemia in the acute phase. As for treatment, one case with pregnancy was treated surgically, with termination of the pregnancy and resection of the pancreas, while the other six were treated by conservative treatment only.
There was a relatively lower incidence of hyperlipidemic pancreatitis in Korea compared with that in the West. The lower fat diet than the typical Western diet may explain the lower incidence of hyperlipidemic pancreatitis in Korea. Nevertheless, the westernization of the Korean dietary habit is increasing the incidence of hyperlipidemic pancreatitis. The low level of attention toward this disease has possibly resulted in few case reports and all the cases, except the pregnant case and the acromegaly case, have recurred frequently. So, accurate and rapid diagnosis, along with early intensive therapy, are essential to treat the recurrence of hyperlipidemic pancreatitis. To prevent the recurrence of hyperlipidemic pancreatitis in diabetes patients, the necessary steps include lower lipid diet education, encouraging exercise, strict blood sugar control and medication of lipid lowering agents.
Figures and Tables
References
1. Carmeron JL, Capuzzi DM, Zuidemia GD, Margolis S. Acute pancreatitis with hyperlipidemia: the incidence of lipid abnormalities in acute pancreatitis. Ann Surg. 1973. 177:483–489.
2. Fredrickson DS, Lees RS. A system for phenotyping hyperlipoproteinemia. Circulation. 1965. 31:321–327.
3. Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990. 19:783–791.
4. Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973. 52:1544–1568.
5. Rose HG, Kranz P, Weinstock M, Juliano J, Haft JI. Inheritance of combined hyperlipoproteinemia: evidence for a new lipoprotein phenotype. Am J Med. 1973. 54:148–160.
6. Nikkila EA, Aro A. Family study of serum lipids and lipoproteins in coronary heart disease. Lancet. 1973. 1:954–959.
7. Thannhauser SJ, editor. . Lipidoses, diseases of the intracellular lipid metabolism. 1958. 3rd ed. New York: Grune & Stratton;307.
8. Farmer RG, Winkelman EI, Brown HB, Lewis LA. Hyperlipoproteinemia and pancreatitis. Am J Med. 1973. 54:161–165.
9. Dominguez-Munoz JE, Malfertheiner P, Ditschhneit HH, Blanco-Chavez J, Uhl W, Buchler M, Ditschuneit H. Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset and severity of the disease. Int J Pancreatol. 1991. 10:261–267.
10. Dominguez-Munoz JE, Junemann F, Malfertheiner P. Hyperlipidemia in acute pancreatitis. Cause or epiphenomenon? Int J Pancreatol. 1995. 18:101–106.
11. Havel RJ. Approach to the patient with hyperlipidemia. Med Clin North Am. 1982. 66:319–333.
12. Carmeron JL, Capuzzi DM, Zuidemia GD, Margolis S. Acute pancreatitis with hyperlipidemia. Evidence for a persistent defect in lipid metabolism. Am J Med. 1974. 56:482.
13. Ohmoto K, Neishi Y, Miyake I, Yamamoto S. Severe acute pancreatitis associated with hyperlipidemia: Report of two cases and review of the literature in Japan. Hepatogastroenterology. 1999. 46:2986–2990.
14. Cho YU, Choi YS, Kim KR, Ko SK, Lee KW. Acute pancreatitis of pregnancy with fatty liver and hyperlipidemia. Inha Med J. 1995. 2:155–159.
15. Lee YG, Choi JS, Kim SH, Lee HJ, Chung MK, Kim CS. A case of acute pancreatitis with hyperlipidemia. Korean J Med. 1986. 31:414–419.
16. Song IB, Kim BM, Lee DY, Kim YJ, Han SS, Kim KH. A case of recurrent pancreatitis with hyperlipidemia in diabetics. J Korean Diabetes Assoc. 1987. 11:195–202.
17. Shin OS, Shin KC, Lee BS, Jang JG, Jung HS, Jung TH, Cho YK, Oh YG. A case of hypertriglyceridemia associated with recurrent pancreatitis. Korean J Med. 1993. 44:854–857.
18. Huang DB, Raskin P. Diabetic hypertriglyceridemia-induced acute pancreatitis masquerading as biliary pancreatitis. J Diabetes Complications. 2002. 16:180–182.
19. Jang GJ, Chung YS, Jun DW, Park SW, Lim SK, Lee HC, Huh KB. Type V Hyperlipoproteinemia during acute pancreatitis in diabetic and recurrent pancreatitic patient. J Korean Diabetes Assoc. 1993. 17:225–238.
20. Lee CY, Lee MY, Lee SY, Hong SN, Kim HH, Kang BH, Kang Hw, Lee BW, Park YJ, Min YK, Lee MS, Kim KW, Kim JH. A case of acromegaly with diabetic ketoacidosis and hypertriglyceridemia-induced acute pancreatitis. J Korean Soc Endocrinol. 2002. 17:110–116.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download