Abstract
Percutaneous approaches, such as percutaneous ethanol injection and radiofrequency ablation, have been most widely used for hepatocellular carcinoma patients who were not eligible for surgery. New technologies to improve the efficacy are currently needed. 166Holmium is a neutron activated radionuclide, and has several beneficial radiophysical characteristics for internal radiation therapy. 166Holmium-Chitosan complex, in which chitosan is chelated with 166Holmium, was developed as a radiopharmaceutical for cancer therapy. We have conducted a pilot study to evaluate the clinical efficacy of transarterial administration of 166Holmium-Chitosan complex in patients with a single and small (< 3 cm) hepatocellular carcinoma. 166Holmium-Chitosan complex, at a dose of 20 mCi per cm of tumor mass-diameter, was administered through the artery that directly fed the tumor. Twelve patients were treated with a median follow-up duration of 26 (range: 12-61) months. The tumor diameter ranged between 1.5 and 2.5 cm. Ten patients (83%) had complete response and two (17%) had partial response. The median complete response duration was not reached. The median AFP level declined from 83.8 to 8.3 ng/mL within 2 months after treatment. No grade III/IV toxicity was observed. Grade I and II toxicities were observed in four patients (2 abdominal pain, 1 fever, and 1 AST/ALT elevation). No toxic death occurred. This preliminary study shows a promising and durable complete response rate with an acceptable safety profile. Further studies with greater accrual of patients are warranted.
Hepatocellular carcinoma (HCC) is a major cause of morbidity and mortality worldwide.1 Percutaneous approaches, such as percutaneous ethanol injection and radiofrequency ablation, have been most widely used for hepatocellular carcinoma patients who were not eligible for surgery.1 Results in large series have demonstrated that complete tumor necrosis can be achieved in approximately 80 to 90% of cases.2,3 These procedures are now considered the gold standard for patients with HCC smaller than 3 cm, who refused or were poor candidates for surgery. However, percutaneous treatments require several treatment sessions to achieve high response rate, and do not provide outcomes comparable to surgical treatments.
Internal radiation therapy using 166Holmium (166Ho) is a unique cancer treatment modality.4-6 166Ho is a neutron activated radionuclide derived from natural 165Holmium, and has several beneficial radiophysical characteristics for internal radiation therapy; it has an appropriate half-life of 26.8 h, a high beta-energy (1.85 MeV), and a low gamma-energy (0.08 MeV) that is readily detectable by gamma camera. The radioactive 166Ho loaded poly (L-lactic acid) microsphere has been investigated in several pre-clinical studies, and results have indicated its potential as a internal radiation therapy for HCC.4-6
Chitosan, the carrier of 166Ho in this study, is a polymer of 2-deoxy-2-amino-D-glucose with β-1,4 bonds; it is derived by alkaline deacetylation of chitin obtained from living crustacean exoskeletons, such as crab shells, shrimps, or cuttlebone. Previous studies have demonstrated that chitosan is non-toxic and bio-absorbable, and has excellent biocompatibility.7,8 166Holmium-Chitosan complex, in which chitosan is chelated with 166Ho, was developed as a radiopharmaceutical for cancer therapy by the Korea Atomic Energy Research Institute (Daejeon, Korea) and Dong Wha Pharmaceutical Company (Kyunggi-do, Korea). Direct administration of the 166Holmium-Chitosan complex into the neutral or basic conditions of a tissue lesion converts the solution into a gel form, and the radioactivity of 166Ho destroys the tumor.9 We have previously reported trans-arterial administration of 166Holmium-Chitosan complex as a novel and effective modality in the treatment of single and large (tumor size ≥ 3 cm in diameter) HCC.10
Under these backgrounds, we have conducted a pilot study to evaluate the clinical efficacy of trans-arterial administration of the 166Holmium-Chitosan complex in patients with a single and small (< 3 cm) HCC who refused or were poor candidates for surgery.
A clinical diagnosis of HCC was established by concomitant positive findings on two out of three imaging techniques, including liver spiral CT, dynamic MRI, and hepatic artery angiography, or by a single positive imaging technique showing hypervascularization associated with an AFP level greater than 400 ng/ml in patients who had at least one risk factor for HCC, such as liver cirrhosis, positive HBV or HCV. Inclusion criteria included: (a) Single HCC < 3 cm; (b) patients who refused or were poor candidates for surgery; (c) ECOG 0-2; (d) an adequate hematologic (neutrophil count ≥ 1,500/µL, platelet count ≥ 100,000/µL), renal function (serum creatinine level ≤ 1.5 times the upper normal limit), liver function (AST and ALT ≤ 5 times the upper normal limit, bilirubin ≤ 4.0 mg/dL), and clotting profile (prothrombin time ≥ 60%); and (f) an expected survival of at least 3 months. Exclusion criteria included: (a) arterio-portal or arteriovenous shunt on angiography; (b) portal vein thrombosis; (c) prior history of upper abdomen radiation; (d) chemotherapy within the 4 weeks prior to our treatment; (e) pregnant or lactating women; and (f) uncontrolled cardiac, pulmonary, or infectious disease. Informed consent was obtained from all patients at the time of enrollment.
Holmium solution [166Ho(NO3)3·5H2O] was obtained from the Korea Atomic Energy Research Institute (Daejeon, Korea); 165Ho was converted to 166Ho in a nuclear reactor at a neutron flux of 1 × 1013 n·cm-1·sec-1. Chitosan was prepared by the Pharmaceutical Development Lab (Dong Wha Pharm Ind. Co., Ltd., Anyang, Korea) and lyophilized before use by evaporation of acetic acid solvent; its molecular weight was 700,000. 166Holmium-Chitosan complex was formed by chemical reaction under a pH 3 solution state. On the day of use, 166Holmium-Chitosan complex was prepared by mixing the holmium and chitosan solutions.
The delivery dose to the hepatoma was estimated by the Monte Carlo simulation using EGS4.11 166Ho was assumed to be uniformly distributed inside the tumor by intra-arterial injection. The surrounding normal tissue was assumed to be source free. Table 1 shows the dose values averaged over the whole tumor volume (mean dose), the outer spherical shell of 0.25 mm thickness (peripheral dose), and the remaining inner sphere (central dose). The central dose ranged from 112 to 120% of the mean dose, whereas the peripheral dose ranged from 88 to 92%.
Selective hepatic angiography was performed by the Seldinger technique. After microcatheter introduction into the super-selected artery that fed the tumor, we slowly injected the 166Holmium-Chitosan complex, at a dose of 20 mCi/mL (740 MBq) per cm of tumor diameter. NaHCO3 (sodium bicarbonate) was empirically administered in an effort to maintain serum alkalization and thus reduce the leaching of the complex into the systemic circulation. Peripheral blood (5 mL) was taken 15 minutes post procedure to measure radioactivity using a beta ray counter. Whole body and liver images were obtained using an ADAC Genesys gamma camera (ADAC Laboratories, Milpitas, USA) immediately after, and also 24 hours post procedure, to analyze the complex distribution. Hydration and G-CSF support was performed at the physician's discretion, depending on the serum beta radiation concentration level. Blood studies (CBC with differential, serum creatinine, AST, ALT, total bilirubin, electrolyte, calcium, phosphorus, total protein and albumin) were performed daily for the first five days after radiation, and also on days 28 and 60; urine pH was assessed daily for the first three days for serum alkalization. Liver spiral CT scan and/or angiography as well as AFP levels were assessed 4 and 8 weeks after the procedure to evaluate response. Thereafter, repeat CT scan was performed every 3 to 4 months.
Response evaluation was performed by either liver spiral CT scan or angiography, and was based on the worst of the two findings. Treatment response was defined by WHO criteria, with the extent of tumor necrosis being seen as mass reduction. A complete response (CR) was defined as complete disappearance of staining on angiographic evaluation and as complete necrosis without a viable portion on spiral CT scan. A partial response (PR) was defined as ≥ 50% decrease in the sum of the bidimensional measurements in both studies. CR duration was defined from the time of CR achievement to disease recurrence. If CT scans obtained at 1 month showed persistence of residual tumor, or if the tumor relapsed on follow-up study, an additional treatment, such as TACE or transarterial chemoinfusion (TACI), was performed at the physician's discretion. Adverse events were assessed using NCI-CTC software (version 2.0).
Between November 1999 and January 2004, a total of 12 patients (9 men and 3 women) entered the study. All patients were diagnosed with HCC on a clinical basis, and were referred to interventional radiology for initial therapy. All patients, except one with Child-Pugh C liver cirrhosis, had operable HCC at the time of diagnosis, however refused surgery. The median follow-up duration was 26 (range: 12-61) months. The patient characteristics are listed in Table 2. The median patient age was 55 (range: 39-72) years. Of the 12 patients, 8 and 4 had HBV or HCV- related HCC, respectively. Liver cirrhosis was present in 4 patients (3 Child-Pugh A, 1 Child-Pugh C); there was no clinical evidence of liver cirrhosis in the other patients. All patients except one had an ECOG performance status of 0 or 1. The median pretreatment AFP level was 84 ng/mL (range: 3-564). The median tumor size was 2.0 cm (range: 1.5-2.5). In 5 patients, the tumor was located on the liver dome.
The median dose of 166Holmium-Chitosan complex administered was 30 mCi (range, 30-50). All patients received a planned dose of 20 mCi/mL per cm of tumor diameter. Treatment caused tumors to appear necrotic on CT scan and to appear avascular on angiography (Fig. 1 and 2). CR was seen in 10 patients (83%), 2 experienced relapse at 6 and 17 months, respectively; both patients received further treatment with TACI and thereafter, remained disease-free for 25 months and 4 months. Median CR duration was not reached. PR was seen in 2 patients (17%), they received additional treatments with TACE. In one patient, additional TACE provided CR and a disease free state thereafter. Of all the patients, 10 are currently alive. One patient, who initially presented as Child-Pugh C liver cirrhosis and showed CR, died of advanced liver cirrhosis 18 months after treatment. The cause of death did not appear to be related to the treatment. The other death was in a patient showing PR, who died of tumor progression 51 months after treatment.
In all patients, AFP levels fell between 99 to 17% of the pretreatment levels (Fig. 3). A drop of 50% or more occurred in 8 of 12 patients. The greatest drop of 99% occurred in a 54 year old female with a 2 cm tumor; she is still alive without recurrence with a follow-up period of 34 months. The median AFP level dropped from 83.8 to 8.3 ng/mL within 2 months after treatment.
The biodistribution following the trans-arterial administration of the complex demonstrated that most of the injected radioactivity localized within the liver and that the extra-hepatic leakage was negligible at 24 hours after treatment (Fig. 4).
In our study, no grade III to IV toxicity was observed in terms of hematologic and nonhematologic toxicities. Only grade I to II nonhematologic toxicities, including two abdominal pain of grade II, one fever of grade I, and one AST/ALT elevation of grade I, were observed. They required only supportive care. No toxic death occurred.
The use of intra-arterially administered radionuclide for treatment of HCC has the potential to overcome the disadvantages of external beam radiation, which is currently limited by the radio sensitivity of healthy tissue. Selective internal radiation treatment with 90Yttrium microspheres has been used as palliative treatment of inoperable HCC or metastatic liver tumors.12,13 However, the currently used radioactive 90Yttrium microspheres cannot be visualized directly with scintigraphy because 90Yttrium is a pure beta emitter. For this reason, estimation of arterio-venous shunting by Tc-MAA scan is required in order to avoid serious complications, such as radiation pneumonitis and gastrointestinal bleeding.14
To overcome this major drawback, radioactive 166Ho was used in our study; it emits both gamma rays for diagnostic scintigraphic imaging and beta particles for therapy. Since holmium is paramagnetic, it can also be visualized clearly with MR imaging. Furthermore, production of 166Ho is simple and less expensive compared with 90Y or other radionuclides, since it comes from a naturally abundant element. Several preclinical studies have shown that intra-arterial administration of 166Holmium poly (L-lactic acid) microspheres has potential for internal radiation therapy of HCC.4-6
Instead of using poly lactic acid microsphere as a vehicle, we used chitosan in our study. Chitosan, a polymer of 2-deoxy-2-amino-D-glucose obtained by deacetylation of chitin, forms a chelate with heavy metals. One major advantage of using chitosan as a carrier is its variable viscosity, which depends on the environmental pH. That is, chitosan dissolves in water to make a clear solution under acidic conditions, but converts to a gel state under basic conditions.9 This unique characteristic confers superiority over other delivery systems, because chitosan functions not only as a drug delivery system, but also through the embolization effect on the tumor's feeding arteriole. In a phase I/IIa clinical trial, 166Ho-chitosan complex was injected percutaneously into a small HCC, resulting in 77.5% complete tumor necrosis and minimal toxicity.15 With this in mind, we conducted the pilot study to evaluate the clinical efficacy of transarterial administration of 166Holmium-Chitosan complex in patients with a single and small (< 3 cm) HCC. In our study, all patients with advanced liver cirrhosis, except one, had resectable HCC but refused surgery.
The main findings of this study are that 83% of patients showed CR and 17% showed PR. This response rate is quite comparable to PEI or RFA. Although both percutaneous treatments provide CR in 80 to 90% of patients, they are highly dependent on the practitioner's skills and techniques. Furthermore, several treatment sessions are required to achieve a high response rate.2,3 Of 5 tumors in the liver dome, 4 showed CR. This analysis is important because percutaneous approach to the liver dome is difficult and treatments are usually considered unsafe in these patients.
A major concern regarding trans-arterial radiotherapy is radionuclide leakage into the systemic circulation. Pharmacokinetic study showed that 166Ho itself cannot be retained in the administration site, unless it is in a chelated complex with chitosan.16 Furthermore, distribution of the radioactivity in tissues, determined by gamma camera, confirmed that most administered radioactivity was retained in the tumor.
Localization of the catheter tip in the super-selected artery that directly feeds the tumor, instead of the hepatic artery proper, increased the selectivity of treatment. In our study, no patients developed radiation-induced pneumonitis or gastritis, which can result from significant shunting to the lung or upper gastrointestinal tract. This was likely due to the fact that patients with vascular shunts were excluded and because of super selection of the tumor-feeding artery.
The minimal radionuclide leakage and super selection of the tumor-feeding artery seemed to be translated into an absence of serious systemic toxicities in our patients. Mild postembolization syndrome, characterized by fever and abdominal pain, was the only adverse event of this treatment.
According to the Monte-Carlo code simulation technique, a dose of 20 mCi 166Ho per cm of tumor diameter was predetermined to expose the normal tissue, in a 3 mm radius, to a radiation dose of 100 Gy. Unfortunately, a volume-based dose escalation could not be evaluated because the drug was too viscous to administer as a large amount. Because of this characteristic, along with toxicity concern the dose was increased according to increasing diameter (20 mCi/cm), but not to tumor volume.
In conclusion, the results of this study demonstrate that trans-arterial 166Holmium-Chitosan complex administration is an effective treatment option with acceptable toxicities for small HCC (< 3 cm). The high CR rate may permit this treatment as one of the curative treatment modalities. However, further trials in a larger patient population are warranted, in order to evaluate the therapeutic efficacy of the 166Holmium-Chitosan complex.
Figures and Tables
Fig. 1
CT scan (A) and angiography (B) of a 2 cm hypervascular HCC (arrow) before trans-arterial 166Holmium-Chitosan complex.
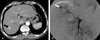
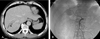
Fig. 4
Gamma scan obtained immediately after treatment shows 166Holmium-Chitosan complex retained within tumor mass.

References
1. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003. 362:1907–1917.
2. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radio-frequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004. 127:1714–1723.
3. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.
4. Nijsen JF, Zonnenberg BA, Woittiez JR, Rook DW, Swildens-van Woudenberg IA, van Rijk PP, et al. Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: effects of preparation and neutron activation techniques. Eur J Nucl Med. 1999. 26:699–704.
5. Mumper RJ, Ryo UY, Jay M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med. 1991. 32:2139–2143.
6. Nijsen F, Rook D, Brandt C, Meijer R, Dullens H, Zonnenberg B, et al. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: a biodistribution study. Eur J Nucl Med. 2001. 28:743–749.
7. Muzzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G, Gazzanelli G, et al. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988. 9:247–252.
8. Hirano S, Noishiki Y. The blood compatibility of chitosan and N-acylchitosans. J Biomed Mater Res. 1985. 19:413–417.
9. Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res. 1997. 34:21–28.
10. Sohn JH, Lee JT, Lee JD, Chung HC, Kim JH, Yoo NC, et al. Transarterial injection of Holmium-166-Chitosan complex (Hol-166) in the treatment of single and large hepatocellular carcinoma: A novel therapeutic modality. ASCO Annual Meeting Proceedings. 2004. 22:4023.
11. Nelson WR, Hirayam H, Rogers DWO. The EGS 4 code system. 1985. Standard Linear Accelerator Center, SLAC;256.
12. Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994. 70:994–999.
13. Salem R, Lewandowski R, Roberts C, Goin J, Thurston K, Abouljoud M, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004. 15:335–345.
14. Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995. 33:919–924.
15. Kim JK, Han KH, Lee JT. The long term therapeutic efficacy and the safety of percutaneous holmium injection for the treatment of small hepatocellular carcinoma. J Hepatol. 2003. 38:Suppl 2. 6.
16. Suzuki YS, Momose Y, Higashi N, Shigematsu A, Park KB, Kim YM, et al. Biodistribution and kinetics of holmium-166-chitosan complex (DW-166HC) in rats and mice. J Nucl Med. 1998. 39:2161–2166.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download