Abstract
Objective
To investigate the diagnostic yield of contrast-enhanced computed tomography (CT) in Crohn's disease (CD) patients presenting with acute severe lower gastrointestinal bleeding (LGIB), and the role of CT in predicting the risk of rebleeding.
Materials and Methods
A consecutive series of 110 CD patients presenting with acute severe LGIB between 2005 and 2016 were analyzed. Among them, 86 patients who had undergone contrast-enhanced CT constituted the study cohort. The diagnostic yield of CT for detecting contrast extravasation was obtained for the entire cohort and compared between different CT techniques. In a subgroup of 62 patients who had undergone CT enterography (CTE) and showed a negative result for extravasation on CTE, the association between various clinical and CTE parameters and the risk of rebleeding during subsequent follow-up was investigated using Cox regression analysis.
Results
The diagnostic yield of CT was 10.5% (9 of 86 patients). The yield did not significantly differ between single-phase and multiphase examinations (p > 0.999), or between non-enterographic CT and CTE (p = 0.388). Extensive CD (adjusted hazard ratio [HR], 3.27; 95% confidence interval [CI], 1.09–9.80; p = 0.034) and bowel wall-to-artery enhancement ratio (adjusted HR, 2.81; 95% CI, 1.21–6.54; p = 0.016) were significantly independently associated with increased rebleeding risks, whereas anti-tumor necrosis factor-α therapy after the bleeding independently decreased the risk of rebleeding (adjusted HR, 0.26; 95% CI, 0.07–0.95; p = 0.041).
Acute severe lower gastrointestinal bleeding (LGIB) is an uncommon but potentially life-threatening complication of Crohn's disease (CD), with reported incidence ranging from 0.6% to 6% (12345678). Moreover, the risk of later rebleeding is fairly high, ranging from 19% to 41% according to the literature (12589). Diagnostic evaluation of LGIB in CD patients is challenging because of several factors. It is often difficult to determine the precise site of bleeding unless active bleeding is directly observed during the examination owing to the presence of multiple areas of inflammation (58). When ileocolonoscopy fails to reveal the bleeding focus, the small bowel is difficult or even impossible to access with video capsule endoscopy or deep enteroscopy in the presence of bowel strictures (1011).
Contrast-enhanced abdominopelvic computed tomography (CT), either routine CT or CT enterography (CTE), is generally recommended in patients with LGIB (1112). However, it has rarely been addressed, if the same recommendation applies to CD patients with LGIB, for whom the presence of CD poses unique difficulties to the CT evaluation. Unlike in other causes of LGIB, for which CT detection of a potential source of bleeding (such as an abnormal vasculature/hyperenhancement or a tumor) instead of direct visualization of bleeding is essentially diagnostic, a direct demonstration of contrast extravasation is generally needed for CT to determine the site of LGIB in CD. As CD patients typically already have multiple sites of mural abnormalities accompanied by hyperenhancement (a common finding of CD inflammation), it is impossible to know whether any such abnormalities are a source of bleeding unless one directly sees bleeding at the time of CT scan. Therefore, the diagnostic impact of CT might not be as apparent in CD patients presenting with LGIB as in patients with other causes of LGIB. Furthermore, as CD patients are generally young, radiation exposure is a concern in recommending a CT examination. Magnetic resonance enterography is currently widely used along with CT for evaluating CD patients (12). Although the use of magnetic resonance imaging to evaluate suspected small-bowel bleeding has been reported, supporting data are scarce; moreover, magnetic resonance imaging is difficult to use in emergency cases such as acute severe LGIB (12). In contrast, given that LGIB in CD patients is most likely related to CD itself rather than other specific causes and, therefore, its management often requires considering the state of CD and the risk of future rebleeding besides hemostatic treatment of the current LGIB (12), performing a CT examination, especially using enterographic technique, might potentially be useful as it provides information about the state of CD and, furthermore, if it could suggest the risk of later rebleeding. Studies on the risk factors for later rebleeding in CD patients presenting with LGIB are scarce (1) and, to our knowledge, there are no related studies on the role, if any, of radiologic imaging.
For these reasons, it would be clinically meaningful to determine the diagnostic value of CT imaging specifically in CD patients presenting with LGIB. The purpose of this study was to investigate primarily the diagnostic yield of contrast-enhanced CT (i.e., direct CT visualization of bleeding) in CD patients presenting with acute severe LGIB and secondarily any additional role of CT in predicting the risk of rebleeding at later follow-up, even if not directly demonstrating bleeding. The knowledge would help guide a more appropriate evidence-based use of CT in the management of CD patients presenting with LGIB.
This retrospective observational study was approved by the Institutional Review Board of Asan Medical Center. The requirement for informed consent was waived.
Crohn's disease patients who presented with acute severe LGIB between January 2005 and December 2016 at Asan Medical Center, a tertiary academic center, were identified through a search of the institutional inflammatory bowel disease registry, by using the eligibility criteria as explained below. The characteristics of the registry have previously been detailed (13). In brief, each new patient with inflammatory bowel disease is registered prospectively and relevant data including baseline patient characteristics, changes in disease behavior or location, development of complications, usage patterns of medications, and dates and types of surgery are continuously updated prospectively. The inclusion criteria for the current study were age ≥ 18 years and CD with acute severe LGIB. The diagnosis of CD was confirmed on the basis of clinical presentation and a combination of endoscopic, histological, radiological, and/or biochemical criteria (1415). Acute severe LGIB was defined as profuse rectal bleeding that 1) resulted in an abrupt decrease in hemoglobin level to < 9 g/dL or to at least 2 g/dL below the baseline, or 2) required transfusion of at least two units of blood within 24 hours (2359). The exclusion criteria were postsurgical bleeding occurring within 30 days after surgery, to avoid including cases of surgery-related (instead of CD-related) bleeding and upper gastrointestinal (GI) bleeding as confirmed with upper endoscopy. According to the eligibility criteria, 110 consecutive patients were identified. Among them, 86 patients (mean age ± standard deviation [SD], 35.1 ± 10.5 years), consisting of 67 men (mean age ± SD, 34.2 ± 9.4 years) and 19 women (mean age ± SD, 38.3 ± 10.0 years), underwent contrast-enhanced CT of the abdomen and pelvis at the time of the LGIB and were included in our study (Fig. 1, Table 1). All patients presented to the emergency department and underwent CT after achieving hemodynamic stability (1–12 hours after the visit).
CT examinations were conducted using 16-, 32-, or 64-slice multidetector scanners (Siemens Medical Solutions, Erlangen, Germany and GE Healthcare, Miwaukee, WI, USA) after intravenous bolus administration of 120–150 mL of 320–370 mg I/mL contrast at a rate of 3 mL/s. Eighteen patients underwent non-enterographic examination (single portal-phase acquisition in 14 patients and dual arterial- and portal-phase acquisitions in 4 patients), whereas 68 patients underwent CTE after an oral administration of 1200 mL of 2.5% sorbitol (single enteric-phase acquisition in 28 patients and dual arterial- and portal-phase acquisitions in 40 patients). CTE instead of non-enterographic CT was chosen whenever plausible, i.e., hemodynamic stability and patient's tolerance to the oral fluid administration. The image acquisition parameters were as follows: beam pitch, 1; gantry rotation time, 0.5 second; field of view to fit; 120 kVp; and an automated tube current modulation with the quality reference set at 200 mAs and image reconstruction using standard filtered back projection for the Siemens scanners and a dose modulation using the noise index of 12.81 and image reconstruction using 30% adaptive statistical iterative reconstruction for the GE scanners. Images were reconstructed in axial and coronal planes with 3- to 5-mm thickness with no interslice gap.
Medical records were reviewed, and the following patient- and disease-related characteristics were collected: age at presentation with acute severe LGIB (hereinafter referred to as index bleeding); sex; CD characteristics according to the Montreal classification (16), including age at CD diagnosis (≤ 16 years [A1], 17–40 years [A2], and > 40 years [A3]), disease location (ileum [L1], colon [L2], ileocolon [L3], and upper GI [L4]), disease behavior (non-stricturing non-penetrating [B1], stricturing [B2], and penetrating [B3]), and perianal disease modifier (occurrence of perianal fistulae and abscesses); previous history of GI bleeding and bowel resection; and CD medication at the time of index bleeding. Use of mechanical hemostatic treatments for index bleeding (surgical resection, angiographic embolization, or endoscopic hemostasis) and use of anti-tumor necrosis factor (TNF)-α after index bleeding was noted. Any new episodes of acute severe LGIB during the follow-up after the index bleeding and the time (in days) until such event from the CT examination for evaluating index bleeding were recorded. At our institution, uneventful patients were routinely followed up with regular visits to the outpatient clinic, at approximately 2-month intervals (ranging from 1 to 3 months depending on individual patient circumstance), along with CD activity index measurement and laboratory tests. Furthermore, patients with any symptoms and signs requiring further medical attention could visit the emergency department or make additional visits to the outpatient clinic at any time.
Either the original clinical reading (obtained by experienced board-certified abdominal radiologists) or additional retrospective re-analysis of CT images was used in this study appropriately according to the analytic purposes. For the analysis of the diagnostic yield of CT for bowel bleeding, we used the original clinical CT reading to avoid the risk of overestimating the yield which is prone to occur in retrospective reassessment. A positive CT result was defined as contrast extravasation into the bowel lumen, pseudoaneurysm, or non-CD-related bowel abnormalities with a bleeding risk, such as neoplasms or vascular lesions. In addition, a board-certified experienced abdominal radiologist performed a retrospective rereading of the CT examinations without the knowledge of the findings at endoscopy, angiography or surgery to confirm if any positive findings were missed in the original clinical reading. Among patients whose CT showed negative results, findings of those who were examined using CTE (n = 62) (Fig. 1) were retrospectively reanalyzed to explore if any association existed between the CTE findings and the recurrence of acute severe LGIB later during the subsequent follow-up. We limited this image analysis to the CTE technique, as non-enterographic routine CT had limitations in evaluating CD-related bowel findings. A board-certified experienced abdominal radiologist, who was aware that the patients had CD and were examined for acute severe LGIB but was blinded to any other clinical data, performed a retrospective reanalysis of the following findings: disease extent on CTE categorized as non-extensive versus extensive (affecting a total bowel length of > 100 cm regardless of location) CD (17), mural thickness in millimeters, and the quantitative ratio between bowel wall enhancement and mesenteric arterial enhancement according to a method established in published studies (1819). When a dual-phase examination had been performed, the greater measurement was chosen for the analysis (19). The two latter parameters were obtained in the most severely affected bowel segment.
The diagnostic yield of CT for bowel bleeding (i.e., the rate of direct CT visualization of bleeding evidence) was the primary study outcome and was determined on a per-patient basis with its 95% confidence interval (CI). The diagnostic yield was compared between single- and dual-phase examinations, as well as between non-enterographic CT and CTE, by using Fisher's exact test. Secondarily, in the subgroup of 62 patients who underwent CTE and showed negative results for bowel bleeding, multivariable analysis with Cox proportional hazard regression was performed to investigate the association between the CTE findings and the later recurrence of severe LGIB. We considered various clinical characteristics that might be associated with GI bleeding in CD patients according to published studies (12) as covariates. Variables with p values of ≤ 0.25 in univariable analysis were chosen as variables for multivariable analysis. For the multivariable analysis, a stepwise backward elimination method was used. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA). A p value of < 0.05 was considered statistically significant.
The diagnostic yield of CT for bowel bleeding was 10.5% (9 of 86; 95% CI, 5.4–18.9%) using the clinical definition of acute severe LGIB mentioned previously as the reference standard, according to the original clinical reading. There was no additional detection of bowel bleeding at the retrospective reanalysis of the images compared to the original clinical reading. All positive CT results were demonstrations of active contrast extravasation, all in areas of active CD inflammation (Fig. 2), including the jejunum (n = 1), ileum (n = 6), and cecum (n = 2), and there were no cases of CT detection of non-CD-related bowel lesions to indicate potential sources of bleeding. The yield was not significantly different between single-phase (9.5%, 4 of 42) and dual-phase (11.4%, 5 of 44) CT studies (p > 0.999). In all five patients examined with dual-phase CT, contrast extravasation was clearly recognized at both arterial and portal phases. The different yields between non-enterographic CT (16.7%, 3 of 18) and CTE (8.8%, 6 of 68) shown in the study sample did not reach a statistical significance (p = 0.388).
Of the nine patients who had positive results for active bleeding on CT, two patients received surgery, one patient (of two patients who underwent angiography) received angiographic embolization, three patients received endoscopic hemostasis, and three patients received pharmacotherapy alone. LGIB was successfully resolved by treating the extravasation seen on CT in all patients. Among the 77 patients with negative CT results for bowel bleeding, two patients received surgery, one patient (out of eight patients who underwent angiography) received angiographic embolization as angiography successfully revealed a bleeding focus, and two patients received endoscopic hemostasis after endoscopic confirmation of bleeding.
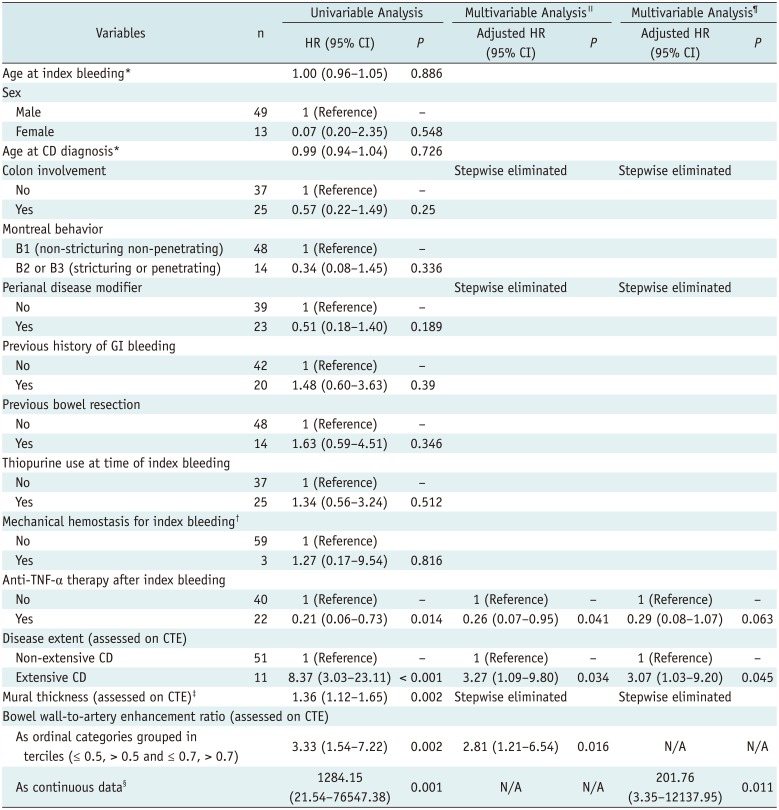
In the subgroup of 62 patients who underwent CTE and showed negative results for bowel bleeding on CTE, severe LGIB recurred in 20 patients (32.3%) during the follow-up (median, 73.3 months; range, 12.7–133.6 months), 2.4–85.6 months after the index bleeding (median, 30.0 months). The cumulative rates of recurred severe LGIB at 1, 3, and 5 years were 4.8%, 22.7%, and 36.6%, respectively (Fig. 3A). The results of Cox proportional hazard regression to identify risk factors associated with recurrence of severe LGIB are presented in Table 3. According to the multivariable analysis accounting for variables listed in Table 3, when the bowel wall-to-artery enhancement ratio was included as ordinal data grouped in terciles, extensive CD as assessed with CTE (adjusted hazard ratio [HR], 3.27; 95% CI, 1.09–9.80; p = 0.034) and greater bowel wall-to-artery enhancement ratio (adjusted HR, 2.81; 95% CI, 1.21–6.54; p = 0.016) were significantly independently associated with increased risks of rebleeding, whereas treatment with anti-TNF-α therapy after the index bleeding independently decreased the risk of rebleeding (adjusted HR, 0.26; 95% CI, 0.07–0.95; p = 0.041) (Table 3, Fig. 3B–D). The results were largely consistent when the bowel wall-to-artery enhancement ratio was considered as continuous data despite somewhat unstable model estimation (Table 3).
In this study, the overall diagnostic yield of contrast-enhanced CT in CD patients presenting with acute severe LGIB was not very high (10.5%, 9 of 86). Nevertheless, even if CT did not reveal the origin of bleeding specifically, a CT examination performed as enterography was still useful as it could help predict the risk of future recurrence of severe LGIB. The diagnostic yield of CT in this study was apparently lower than those reported in more general settings (essentially non-CD) of LGIB. According to one review, the overall diagnostic yield of CT in LGIB patients consisting of mostly non-CD patients was 60%, with the lowest report of 25% (20). Furthermore, several studies reported CTE yields of 24.1% to 26.6% in patients suspected of having small-bowel bleeding (212223). The lower yield of CT in our study is likely related to several factors. First, a direct demonstration of contrast extravasation (instead of bowel abnormalities that can bleed, if not already bleeding at the time of examination) was needed to make a positive call of GI bleeding on CT because of the pre-existing hyperenhancing mural lesions in CD. Second, as patients were examined after achieving hemodynamic stability, many patients might have been in temporary hemostasis state. Third, oral fluid administration used with CTE might have diluted small amounts of extravasated contrast material, thereby decreasing the ability to detect subtle active bleeding. Although the diagnostic yields for LGIB were not significantly different between CTE and non-enterographic CT in this study, the point estimate value was smaller for CTE (8.8% compared with 16.7% for non-enterographic CT). Further confirmation in a larger study would be worthwhile, as this statistical comparison is likely limited owing to the retrospective nature and the small number of patients who underwent non-enterographic CT. Additionally, the low yield may be due to the nature of CD itself, as the previous studies seldom included CD patients. Although the diagnostic yield of CT was low in this study, the localization of bleeding by CT, when achieved, was effective in that LGIB could successfully be resolved by treating the bleeding identified on CT in all patients.
For evaluating GI bleeding, multiphase CT including unenhanced, arterial, and portal phases is generally recommended (1224). Unenhanced images are helpful in differentiating active bleeding from any preexisting intraluminal hyperattenuating pseudolesions. For contrast-enhanced images, combining findings from arterial and portal phases may reveal increase in size and change in morphology of the extravasated contrast with time, which may help recognize active bleeding. By contrast, our study results do not indicate that the diagnostic yield of multiphase CT is superior to single-phase CT for detecting bleeding. Although, in all five CD patients examined with multiphase CT, contrast extravasation was clearly depicted at both arterial and portal phases, all of our study patients had severe bleeding and the difference of multiphase versus single-phase scans may likely be more pronounced in patients with less severe bleeding. Considering these results and the low diagnostic yield of CT, routine use of multiphase CT in CD patients presenting with acute severe LGIB may probably be excessive and such an approach may cause unbeneficial radiation exposure to many patients, which could be a concern considering that CD patients are mostly young and are subject to multiple repeated radiologic examinations. However, these results are limited due to the retrospective comparison and the modest sample size, although the patient characteristics were grossly similar between the two techniques. Further confirmation would be needed.
Both non-enterographic routine CT and CTE can be used to evaluate LGIB depending on patient circumstance (12). The oral administration of neutral fluid contrast used for CTE substantially increases the detection of bowel abnormalities per se compared with non-enterographic CT. However, as mentioned earlier, the fluid may dilute extravasated contrast material, thereby decreasing the ability to detect subtle active bleeding. Therefore, CTE could be disadvantageous in a situation where direct visualization of contrast extravasation is needed, like CD patients presenting with bleeding. One potential advantage of performing CTE in CD patients presenting with acute severe LGIB is obtaining information to predict the risk of future rebleeding. Considering that acute severe LGIB is a potentially life-threatening complication and that recurrence is not rare in CD patients, estimating the risk of future rebleeding would be important. Our study is seemingly the first study to explore CTE findings as predictors of occurrence of future rebleeding in CD patients presenting with acute severe LGIB. Both extensive CD as assessed with CTE and greater bowel wall-to-artery enhancement ratio were independent predictors for the risk of rebleeding. The etiology of severe LGIB in CD patients has not been fully elucidated; however, one possible explanation is a transmural inflammation leading to the penetration and erosion of large vessels (25). The association between the CTE findings and the risk of rebleeding seems compatible with this theory, as the CTE findings reflect more extensive and more severe inflammation. The advantages and disadvantages of CTE vs. non-enterographic CT for CD patients presenting with acute severe LGIB should be clarified further with more studies to determine the optimal scan techniques for these patients.
Use of anti-TNF-α therapy after the index bleeding was independently negatively associated with the risk of rebleeding in our study. Similarly, there have been reports of the beneficial effects of anti-TNF-α in CD patients presenting with acute severe LGIB (2627282930313233). Anti-TNF-α agents have been shown to induce and maintain mucosal healing (34); thereby, these may treat the bleeding and prevent recurrent bleeding. Our study is also meaningful in that it provides further proof for anti-TNF-α as a therapy for LGIB in CD.
Our study has several limitations. First, it was a retrospective study of modest sample size; therefore, it is prone to potential selection bias. Not all eligible patients underwent CT, and the analysis of CT findings concerning the risk of rebleeding only applies to those who underwent CTE. Second, our results were from a single-center experience. Therefore, further prospective confirmation of the results in a multicenter setting that includes a larger number of patients would be needed.
In conclusion, the diagnostic yield of contrast-enhanced CT was not high in CD patients presenting with acute severe LGIB. Nevertheless, even a negative CTE may likely be beneficial, as it can help predict the risk of later rebleeding. Further studies are needed to determine the optimal CT techniques for these patients.
References
1. Li G, Ren J, Wang G, Wu Q, Gu G, Ren H, et al. Prevalence and risk factors of acute lower gastrointestinal bleeding in Crohn disease. Medicine (Baltimore). 2015; 94:e804. PMID: 25984665.

2. Kim KJ, Han BJ, Yang SK, Na SY, Park SK, Boo SJ, et al. Risk factors and outcome of acute severe lower gastrointestinal bleeding in Crohn's disease. Dig Liver Dis. 2012; 44:723–728. PMID: 22497905.

3. Kostka R, Lukás M. Massive, life-threatening bleeding in Crohn's disease. Acta Chir Belg. 2005; 105:168–174. PMID: 15906908.

4. Veroux M, Angriman I, Ruffolo C, Barollo M, Buffone A, Madia C, et al. Severe gastrointestinal bleeding in Crohn's disease. Ann Ital Chir. 2003; 74:213–215. discussion 216. PMID: 14577120.
5. Pardi DS, Loftus EV Jr, Tremaine WJ, Sandborn WJ, Alexander GL, Balm RK, et al. Acute major gastrointestinal hemorrhage in inflammatory bowel disease. Gastrointest Endosc. 1999; 49:153–157. PMID: 9925691.

6. Driver CP, Anderson DN, Keenan RA. Massive intestinal bleeding in association with Crohn's disease. J R Coll Surg Edinb. 1996; 41:152–154. PMID: 8763176.
7. Cirocco WC, Reilly JC, Rusin LC. Life-threatening hemorrhage and exsanguination from Crohn's disease. Report of four cases. Dis Colon Rectum. 1995; 38:85–95. PMID: 7813353.
8. Robert JR, Sachar DB, Greenstein AJ. Severe gastrointestinal hemorrhage in Crohn's disease. Ann Surg. 1991; 213:207–211. PMID: 1998401.

9. Belaiche J, Louis E, D'Haens G, Cabooter M, Naegels S, De Vos M, et al. Acute lower gastrointestinal bleeding in Crohn's disease: characteristics of a unique series of 34 patients. Belgian IBD Research Group. Am J Gastroenterol. 1999; 94:2177–2181. PMID: 10445546.
10. Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol. 2006; 101:2218–2222. PMID: 16848804.

11. Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG clinical guideline: diagnosis and management of small bowel bleeding. Am J Gastroenterol. 2015; 110:1265–1287. quiz 1288. PMID: 26303132.

12. Park SH, Ye BD, Lee TY, Fletcher JG. Computed tomography and magnetic resonance small bowel enterography: current status and future trends focusing on Crohn's disease. Gastroenterol Clin North Am. 2018; 47:475–499. PMID: 30115433.
13. Park SH, Yang SK, Park SK, Kim JW, Yang DH, Jung KW, et al. Long-term prognosis of crohn’s disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014; 20:488–494. PMID: 24412992.
14. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6. discussion 16-19. PMID: 2617184.

15. Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. European Crohn's and Colitis Organisation (ECCO). The second European evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis. 2010; 4:7–27. PMID: 21122488.

16. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753. PMID: 16698746.

17. Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. ECCO. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017; 11:3–25. PMID: 27660341.

18. Baker ME, Walter J, Obuchowski NA, Achkar JP, Einstein D, Veniero JC, et al. Mural attenuation in normal small bowel and active inflammatory Crohn's disease on CT enterography: location, absolute attenuation, relative attenuation, and the effect of wall thickness. AJR Am J Roentgenol. 2009; 192:417–423. PMID: 19155404.

19. Kim C, Park SH, Yang SK, Ye BD, Park SH, Lee JS, et al. Endoscopic complete remission of Crohn disease after anti-tumor necrosis factor-α therapy: CT enterographic findings and their clinical implications. AJR Am J Roentgenol. 2016; 206:1208–1216. PMID: 26998628.

20. Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010; 8:333–343. quiz e44. PMID: 20036757.

21. Huprich JE, Fletcher JG, Fidler JL, Alexander JA, Guimarães LS, Siddiki HA, et al. Prospective blinded comparison of wireless capsule endoscopy and multiphase CT enterography in obscure gastrointestinal bleeding. Radiology. 2011; 260:744–751. PMID: 21642417.

22. Lee SS, Oh TS, Kim HJ, Chung JW, Park SH, Kim AY, et al. Obscure gastrointestinal bleeding: diagnostic performance of multidetector CT enterography. Radiology. 2011; 259:739–748. PMID: 21460027.

23. Shin JK, Cheon JH, Lim JS, Park JJ, Moon CM, Jeon SM, et al. Long-term outcomes of obscure gastrointestinal bleeding after CT enterography: does negative CT enterography predict lower long-term rebleeding rate? J Gastroenterol Hepatol. 2011; 26:901–907. PMID: 21073673.

24. Soto JA, Park SH, Fletcher JG, Fidler JL. Gastrointestinal hemorrhage: evaluation with MDCT. Abdom Imaging. 2015; 40:993–1009. PMID: 25637128.

25. Korzenik JR. Massive lower gastrointestinal hemorrhage in Crohn's disease. Curr Treat Options Gastroenterol. 2000; 3:211–216. PMID: 11097738.

26. Belaiche J, Louis E. Severe lower gastrointestinal bleeding in Crohn's disease: successful control with infliximab. Am J Gastroenterol. 2002; 97:3210–3211. PMID: 12492221.

27. Papi C, Gili L, Tarquini M, Antonelli G, Capurso L. Infliximab for severe recurrent Crohn's disease presenting with massive gastrointestinal hemorrhage. J Clin Gastroenterol. 2003; 36:238–241. PMID: 12590236.

28. Tsujikawa T, Nezu R, Andoh A, Saotome T, Araki Y, Ishizuka Y, et al. Inflixmab as a possible treatment for the hemorrhagic type of Crohn's disease. J Gastroenterol. 2004; 39:284–287. PMID: 15065007.

29. Meyer MM, Levine EJ. Acute hemorrhagic Crohn's disease controlled with infliximab. Inflamm Bowel Dis. 2009; 15:1456–1457. PMID: 19107774.

30. Ando Y, Matsushita M, Kawamata S, Shimatani M, Fujii T, Okazaki K. Infliximab for severe gastrointestinal bleeding in Crohn's disease. Inflamm Bowel Dis. 2009; 15:483–484. PMID: 18668673.

31. Julián Gómez L, Atienza R, Barrio J, Gil P, Gómez de la Cuesta S, Pinto P, et al. [Infliximab treatment of severe bleeding complicating Crohn's disease]. Rev Esp Enferm Dig. 2010; 102:57–58. PMID: 20187687.
32. Alcalde Vargas A, Justiniano JM, Carnerero EL, Salado CT, Domingo IG, Galán JL. [Utility of infliximab therapy in severe enterorrhagia associated with Crohn's disease. Report of three cases]. Gastroenterol Hepatol. 2011; 34:24–28. PMID: 21168243.
33. Aniwan S, Eakpongpaisit S, Imraporn B, Amornsawadwatana S, Rerknimitr R. Infliximab stopped severe gastrointestinal bleeding in Crohn's disease. World J Gastroenterol. 2012; 18:2730–2734. PMID: 22690085.

34. D'haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: a European multicenter trial. Gastroenterology. 1999; 116:1029–1034. PMID: 10220494.
Fig. 1
Flow diagram of study patients.
CD = Crohn's disease, CT = computed tomography, LGIB = lower gastrointestinal bleeding
Fig. 2
20-year-old male patient with CD presenting with acute severe LGIB.
Axial (A) and coronal (B) CTE images show contrast extravasation into bowel lumen (arrows), indicating ongoing bleeding in ileal area that shows mural thickening and hyperenhancement, ulcers, and increased vasa recta, which are signs of active Crohn's inflammation. CTE = CT enterography
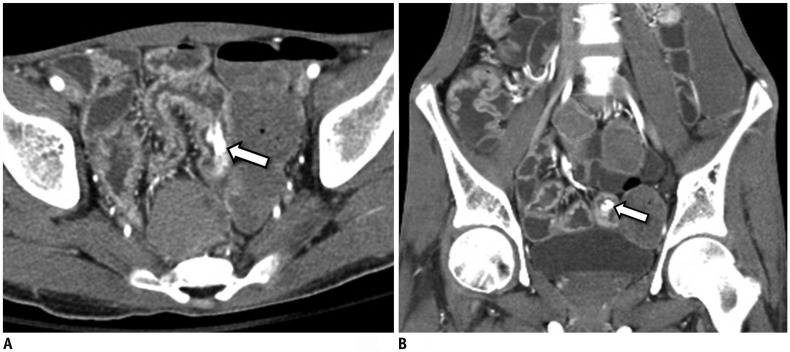
Fig. 3
Cumulative probability of recurred severe LGIB in 62 patients who were examined with CTE and showed negative CTE results for bowel bleeding.
Overall result (A) and results according to disease extent assessed with CTE (B), bowel-to-artery enhancement ratio assessed with CTE (C), and anti-TNF-α therapy after index bleeding (D). CI = confidence interval, HR = hazard ratio, TNF = tumor necrosis factor
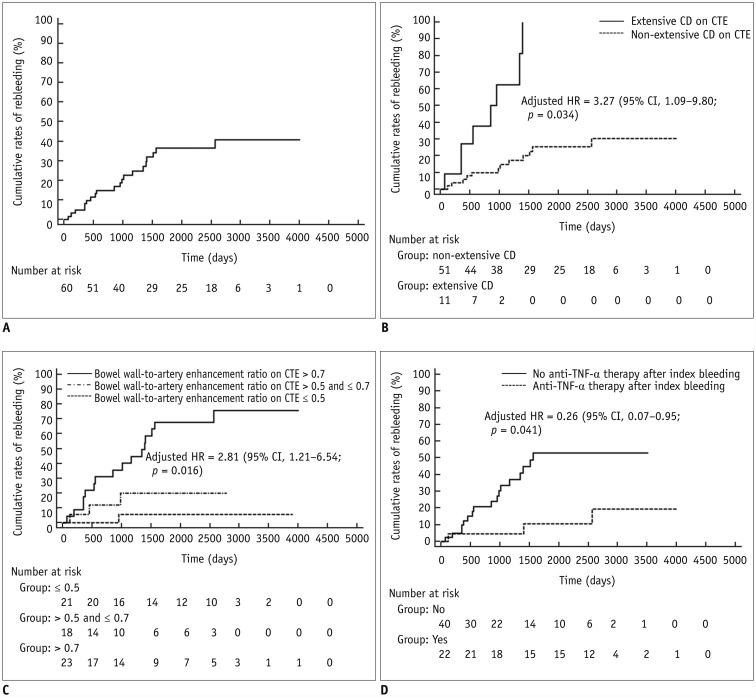
Table 1
Demographics and Clinical Characteristics of Entire Study Population
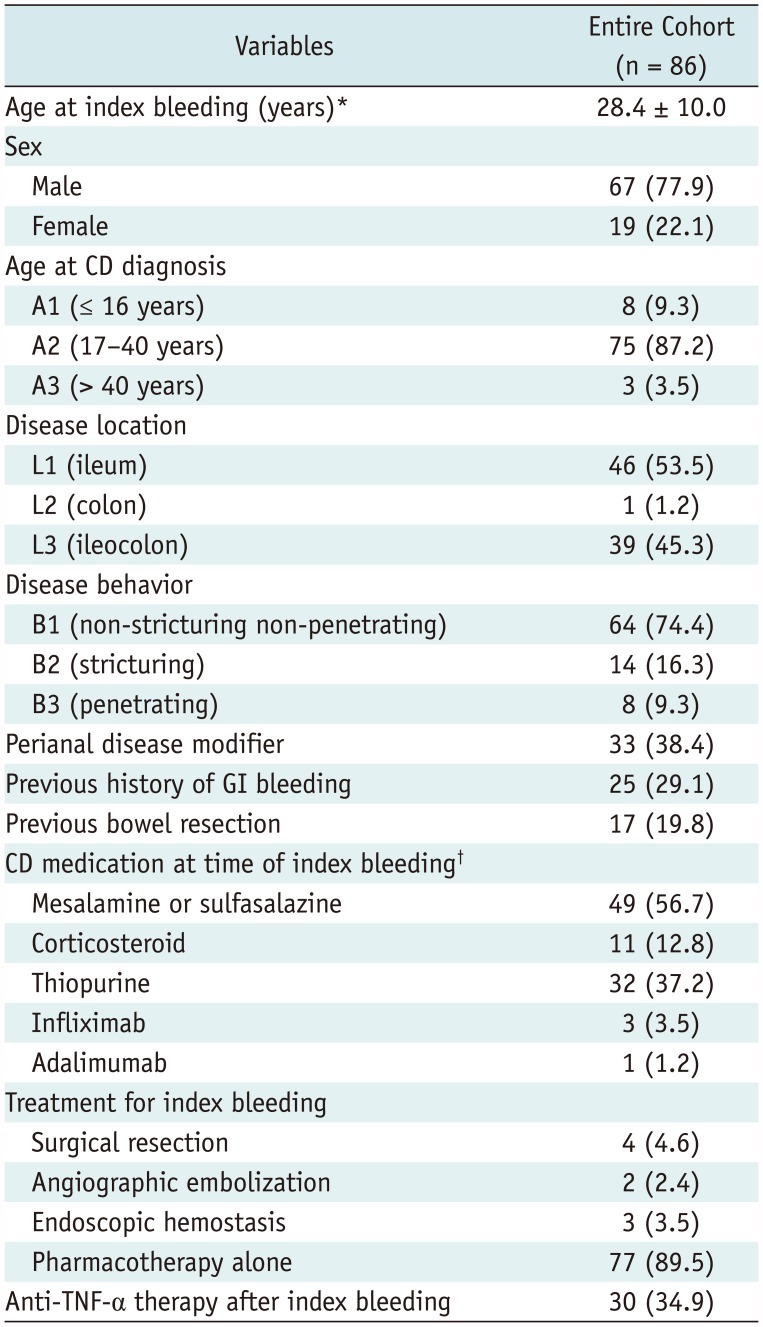
Table 2
Patient Characteristics in Select Subgroups
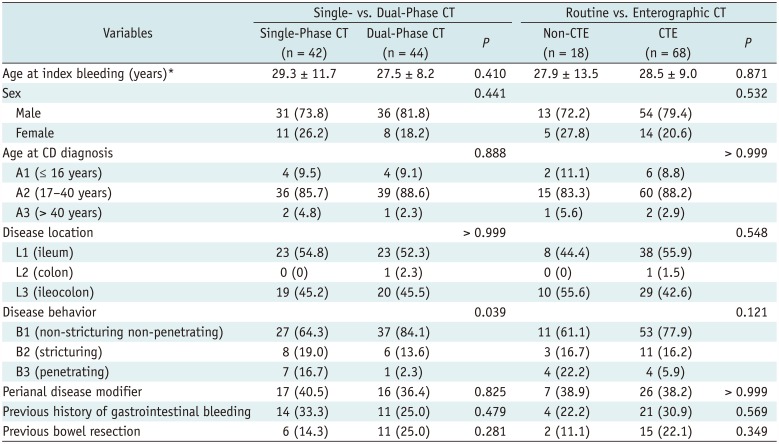
Table 3
Univariable and Multivariable Cox Analyses of Risk Factors for Recurrence of Severe LGIB (for 62 Patients Who Underwent CTE and Showed Negative Results for Bowel Bleeding)
*HR per 1 year increase, †Surgery (n = 2) and angiographic embolization (n = 1), ‡HR per 1-mm increase, §HR per 0.1 increase of index value, ∥Multivariable Cox proportional hazard regression including bowel-to-artery enhancement ratio as ordinal data (grouped in terciles) for more stable statistical modeling given moderate sample size, ¶Multivariable Cox proportional hazard regression including bowel-to-artery enhancement ratio as continuous data. CI = confidence interval, HR = hazard ratio, LGIB = lower gastrointestinal bleeding, N/A = not applicable




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download