Abstract
Objective
To systematically determine the treatment outcomes of percutaneous microwave ablation (MWA) in patients with malignant renal tumor.
Materials and Methods
Original studies that reported the clinical outcomes of MWA in patients with malignant renal tumors were identified in MEDLINE and EMBASE from 2012 to June 30, 2017. Inverse variance and random-effects models were used to evaluate and acquire meta-analytic summary estimates of various clinical outcomes, including technical outcomes (technical success rate [TSR] and technical efficacy rate [TER]), oncologic outcomes (local tumor recurrence rate [LRR], cancer-specific survival rate [CSSR], and overall survival rate [OSR]), and complications.
Results
Among the 145 articles screened, 13 articles including 567 patients carrying 616 malignant renal tumors were included in the meta-analysis. The meta-analytic pooled TSR and TER were 97.3% (95% confidence interval, 94.3–99.4%; I2 = 0.0%) and 97.6% (95.0–99.4%, I2 = 48.5%), respectively. The meta-analytic pooled LRR was 2.1% (0.3–4.7%, I2 = 54.1%). At 1-, 2-, 3-, and 5-year follow-up time points, the meta-analytic pooled CSSRs were 99.1% (97.2–100.0%; I2 = 0.0%), 98.4% (95.1–100.0%; I2 = 31.2%), 97.6% (93.4–99.9%; I2 = 52.3%), and 96.9% (93.3–99.2%; I2 = 0.0%) respectively, while the OSRs were 98.3% (96.1–99.8%; I2 = 0.0%), 94.9% (91.7–97.5%; I2 = 0.0%), 86.8% (81.9–91.1%; I2 = 22.1%), and 81.9% (75.4–87.6%; I2 = 0.0%). In terms of major complications, a 1.8% (0.6–3.3%; I2 = 0.0%) rate of meta-analytic pooled incidence was found.
Renal cell carcinoma (RCC) is the most frequent malignant renal tumor, with 30000 new cases diagnosed every year in the United States alone (1). Surgical resection is the standard treatment for patients with clinical stage T1a RCC, with increasing emphasis on nephron-sparing approaches (23) including open or laparoscopic partial nephrectomy and percutaneous thermal ablations, i.e., radiofrequency ablation (RFA), cryoablation, and microwave ablation (MWA). As percutaneous thermal ablation is minimally invasive, with decreased operative time, and reduced hospital stay and inherent surgical risks, it has become an effective and safe treatment option for renal tumors (4).
Microwave ablation is a heat-based modality with a mechanism of cell death identical to that of other thermal ablation techniques, but which offers several physical advantages associated with the delivery of heat (56). As MWA has several advantages over RFA, including higher intratumoral temperatures, larger ablation volumes, and quicker ablation (7), multiple studies have recently investigated and reported the efficacy and safety of MWA (48910111213141516171819). However, the results were varied, and most of the studies included a small number of patients, with limited precision of the estimated efficacy and safety of MWA.
To overcome these limitations, two meta-analyses of the efficacy and safety of MWA were conducted. One of them included only a single MWA study that compared clinical outcomes between surgical nephrectomy and thermal ablation (20). The other meta-analysis included seven MWA studies: three related to percutaneous image-guided MWA and four involving retroperitoneoscopic MWA (21). This meta-analysis included studies which were published between 2010 and 2012. However, 13 percutaneous MWA studies were published after this meta-analysis (since 2012) (4891011121516171819). Hence, we believe that it is timely and important to establish a systematic summary regarding the efficacy and safety of MWA for the management of malignant renal tumor. The findings of such a study will facilitate current management and evidence-based practices.
Thus, we aimed to systematically determine the treatment outcomes of percutaneous MWA in patients with malignant renal tumor.
This study followed the Meta-analysis of Observational Studies in Epidemiology (22) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (2324). Institutional Review Board approval was not required for this study.
A thorough search of PubMed/MEDLINE and EMBASE databases was conducted to identify original research articles investigating the clinical outcomes of MWA in patients with a malignant renal tumor. The search query was designed to provide a sensitive literature search, to avoid overlooking any relevant articles. Subsequently, a manual evaluation of the searched articles was performed to determine the relevant studies. The following search terms were used: (microwave) AND (RFA OR ablation) AND (carcinoma, renal cell OR RCC OR renal cell carcinoma OR clear cell carcinoma OR renal cancer OR renal tumor OR kidney cancer) AND (recur* OR efficacy OR outcome). No beginning date limit was set. The literature search was continuously updated until the end of June 2017. The search was restricted to human subjects and English-language studies. To expand the search, article bibliographies that were considered valid according to the selection criteria were screened for other potentially suitable articles.
After removing duplicate articles, the remainder were reviewed for the following components to determine eligibility: 1) patients with malignant renal tumor; 2) treatment with percutaneous image-guided MWA and follow-up of the lesions after treatment; 3) no comparison requirements; 4) technical outcomes (technical success rate [TSR] and technical efficacy rate [TER]), oncologic outcomes (local tumor recurrence rate [LRR], cancer-specific survival rate [CSSR], and overall survival rate [OSR]), and complications; and 5) any type of study design.
Further exclusion criteria were: 1) case reports, review articles, editorials, letters, comments, and conference abstracts/proceedings; 2) research articles published before 2012, and 3) studies with overlapping patients and data. In cases involving duplicate data, they were included only once in this study by selecting the study with more comprehensive results. Articles were first screened by their titles and abstracts. The full texts of the articles were reviewed after selecting potentially eligible abstracts. Both steps were performed by two independent reviewers who had expertise in the subject matter and in performing systematic literature reviews. The two reviewers eliminated only those articles that were clearly ineligible. Articles with ambiguity or that generated differences in opinion between the two independent reviewers were re-evaluated through a consensus discussion with a third reviewer (who also had experience in the subject matter and in performing systematic literature reviews).
The following data were extracted onto a predefined data form: 1) study characteristics including authors, year of publication, institution, country of origin, study period, and study design (prospective vs. retrospective); 2) patient characteristics including age, sex, the number of patients, and indication; 3) tumor characteristics including the number of lesions and tumor size; 4) ablation technique; 5) follow-up protocol and follow-up length; and 6) technical outcomes (TSR and TER), oncologic outcomes (LRR, CSSR, and OSR), and complications. Each numerical result was directly extracted when specifically reported. Technical success was defined as correct positioning of the antenna inside the lesion targeted for ablation and subsequent complete ablation, as shown on imaging tests immediately after the MWA session (817). Technical effectiveness was defined as the absence of thermo-ablative residues in contrast-enhanced computed tomography (CT) performed 1 month after MWA treatment (81725). Oncologic outcomes including CSSR and OSR were obtained using Kaplan-Meier curves, or calculated using the follow-up results when this value was not reported. CSSR refers to the proportion of patients who survived or dead for reasons other than RCC after the defined follow-up time, and OSR refers to the proportion of patients who were alive after the defined follow-up time (817). As the studies that directly reported the oncologic results at 1-, 2-, 3-, or 5-year follow-up intervals, the extraction of numerical data was performed at these follow-up time points. Complications were defined as major or minor according to the Society of Interventional Radiology classifications. Two reviewers independently performed data extraction with all discrepancies resolved at a consensus meeting in the presence of a third reviewer.
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of each cohort study. Quality assessment included three broad domains: 1) selection (up to 4 stars), 2) comparability (up to 2 stars), and 3) outcome (up to 3 stars). The maximum score assigned by the NOS is 9 stars. Two reviewers independently evaluated the quality, with all discrepancies resolved through a consensus discussion with a third reviewer not involved with the included studies.
The pooled proportions of the technical and oncologic outcomes, and complications were adopted as the main indices for this meta-analysis to evaluate treatment outcomes of MWA in patients with suspected malignant renal tumor. Technical outcomes included TSR and TER. Oncologic outcomes included LRR, CSSR, and OSR. The CSSR and OSR were assessed at each follow-up time point, i.e., 1, 2, 3, and 5 years. Complications included the incidence of major and minor complications. Subgroup analysis was performed according to the type of image guidance (ultrasonography [US] vs. CT).
For the meta-analysis, the inverse variance method was used to calculate weights. The cumulative incidence and its 95% confidence interval (CI) were obtained using a restricted maximum-likelihood estimation of random effects model. Study heterogeneity was assessed using the Cochrane Q-test and Higgins I2 statistics. A p value < 0.10 in the Q-test indicated substantial heterogeneity. I2 was interpreted as suggested in the literature: 0–25%, might not be important; 25–50%, low heterogeneity; 50–75%, moderate heterogeneity; and 75–100%, high heterogeneity (2627).
Publication bias was visually assessed using a funnel plot, and its statistical significance was tested using a mixed-effects meta-regression model.
R version 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria) with the ‘metafor’ package was used for the analyses.
Our literature search process is outlined in Figure 1. A total of 145 articles were screened after removal of the duplicate articles. Of these 145 articles, 125 were excluded on the basis of their titles and abstracts. Seven additional articles were excluded after reviewing the full text (Supplementary Table 1 in the online-only Data Supplement). Finally, the remaining 13 articles involving 567 patients with 616 malignant renal tumors met the eligibility criteria and were included (Table 1).
All studies had a retrospective study design. Eight studies were reported from Western countries (4891415171819) and five from Eastern countries (1011121316). In nine studies, the mean tumor size was less than 3.0 cm (489101115171819). For technical outcomes, six studies reported TSR (4814151719) and 13 studies reported TER (48910111213141516171819). For oncologic outcomes, 13 studies reported LRR (48910111213141516171819), seven studies reported CSSR (8101213141718), and eight studies reported OSR (810121314151718). All 13 studies reported various major or minor complications post-MWA (48910111213141516171819).
Ultrasonography was used for imaging guidance in five studies (1011121316), CT in five studies (49141519), and both CT and US in three studies (81718). The power of ablation was 50 W or more in eight studies (910111213141618) and 45 W in one study (8), and three studies failed to indicate specifically the details of ablation generator power (41519).
The results of the assessment of study quality are summarized in Supplementary Figure 1 (in the online-only Data Supplement). All studies were well-designed cohorts with moderate-to-high quality according to the NOS assessment (6–8 stars out of 9). As none of the studies included a non-exposed cohort, we could not evaluate the question on selection of the non-exposed cohort. Of the total 13 studies, 8 were of a high quality with more than 7 stars. Within the three domains, a notable area of quality concern was the outcome domain, because of insufficient follow-up duration.
The technical outcomes reported in the 13 individual studies, including TSR and TER, are summarized in Figure 2. The meta-analytic pooled TSR and TER were 97.3% (95% CI, 94.3–99.4%, I2 = 0.0%) and 97.6% (95% CI, 95.0–99.4%, I2 = 48.5%), respectively. No substantial study heterogeneity was observed in either outcome.
The oncologic outcomes reported in the 13 individual studies, including LRR, CSSR, and OSR are summarized in Table 2. the meta-analytic pooled LRR was 2.1% (95% CI, 0.3–4.7%, I2 = 54.1%) (Supplementary Fig. 2 in the online-only Data Supplement). Six of 13 studies reported no local tumor recurrence. The meta-analytic pooled CSSRs at 1-, 2-, 3-, and 5-year follow-up were 99.1% (95% CI, 97.2–100.0%; I2 = 0.0%), 98.4% (95% CI, 95.1–100.0%; I2 = 31.2%), 97.6% (95% CI, 93.4–99.9%; I2 = 52.3%), and 96.9% (95% CI, 93.3–99.2%; I2 = 0.0%), respectively (Fig. 3A). The meta-analytic pooled OSRs at the 1-, 2-, 3-, and 5-year follow-up were 98.3% (95% CI, 96.1–99.8%; I2 = 0.0%), 94.9% (95% CI, 91.7–97.5%; I2 = 0.0%), 86.8% (95% CI, 81.9–91.1%; I2 = 22.1%), and 81.9% (95% CI, 75.4–87.6%; I2 = 0.0%), respectively (Fig. 3B). Moderate study heterogeneity was observed in terms of the 3-year CSSR. However, there was no substantial study heterogeneity in CSSR at the other follow-up time points, or in OSR at any of the time points.
The meta-analytic pooled incidences of major and minor complications were 1.8% (95% CI, 0.6–3.3%; I2 = 0.0%) and 17.5% (95% CI, 5.8–33.4%; I2 = 90.1%), respectively (Fig. 4). The incidence of major complications did not show any substantial heterogeneity, whereas minor complications showed high heterogeneity. The specific major and minor complications are summarized in Table 3.
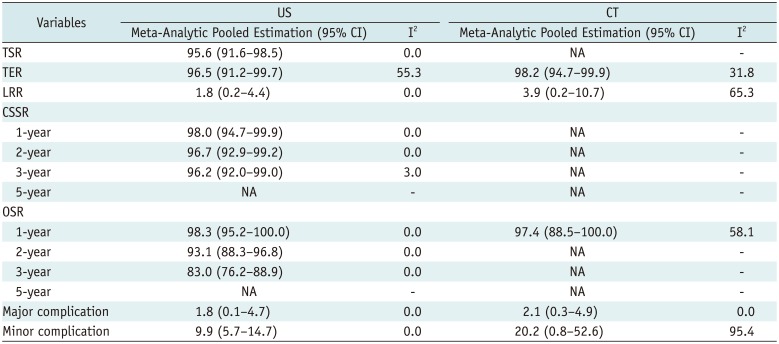
The results for subgroup analysis are summarized in Table 4. The meta-analytical pooled TSR, CSSR, OSR values for CT were not calculated due to the lack of data from 13 included articles. The meta-analytic pooled TER for US was 96.5% (95% CI, 91.2–99.7%; I2 = 55.3%), which was similar to that of CT, 98.2% (95% CI, 94.7–99.9%; I2 = 31.8%). The meta-analytic pooled LRRs for US and CT were 1.8% (95% CI, 0.2–4.4%; I2 = 0.0%) and 3.9% (95% CI, 0.2–10.7%; I2 = 65.3%), respectively. The meta-analytic pooled incidences of major and minor complications for US were 1.8% (95% CI, 0.1–4.7%; I2 = 0.0%) and 9.9% (95% CI, 5.8–14.7%; I2 = 0.0%), respectively, and those for CT were 2.1% (95% CI, 0.3–4.9%; I2 = 0.0%) and 20.2% (95% CI, 0.8–52.6%; I2 = 95.4%), respectively.
No significant publication bias was detected for the various study outcomes, except for the LRR (Supplementary Fig. 3 in the online-only Data Supplement).
This study showed that MWA had overall excellent technical outcomes for 567 patients with 616 malignant renal tumors, with a meta-analytic summary TSR of 97.3% (95% CI, 94.3–99.4%) and TER of 97.6% (95% CI, 95.0–99.4%). MWA also showed good oncologic outcomes, with a meta-analytic summary LRR of 2.1% (95% CI, 0.3–4.7%), and a CSSR of 96.9% (95% CI, 93.3–99.2%) at the 5-year follow-up time point, and an OSR of 81.9% (95% CI, 75.4–87.6%) at the 5-year follow-up time point.
Compared with a previous meta-analysis (21), the present study showed higher TER (97.6% vs. 91.3%), higher CSSR (98.4% at the 2-year follow-up vs. 96.8% at the 18-month follow-up), and lower LRR (2.1% vs. 2.5%), which are attributed to the technical advances in MWA and the use of imaging guidance. Four out of the seven studies included in the previous meta-analysis were retroperitoneoscopic MWA studies without imaging guidance, and three were percutaneous MWA studies with imaging guidance. Accurate delineation of the tumor is necessary for successful percutaneous ablation (11). Therefore, the use of imaging guidance is likely to have improved the technical and oncologic outcomes. In the subgroup analysis of US and CT, image-guided MWA showed similar TERs, although CT tended to have a higher LRR and incidence of complications than US. Although this tendency did not show a statistically significant difference in this meta-analysis, further studies are needed. In addition, various ablation techniques such as gas-cooled microwave antenna, high generator power, and hydro-displacement of non-target anatomy may have improved the efficacy of MWA (428). As the present study included 13 published articles since 2012, our findings facilitate improvement of the current management guidelines and contribute to evidence-based practice.
In previous studies, RFA of renal tumors measuring 4 cm or smaller, had a TER of 91–97% and LRR of 0–23% (293031), while cryoablation of small RCC had a TER of 96.9–100% and LRR of 1.3–5.2% (31323334). Compared with these results, the present study showed that MWA had a similar or slightly better TER and LRR than RFA (97.6% vs. 91–97% for TER; 2.1% vs. 0–23% for LRR) or cryoablation (97.6% vs. 96.9–100% for TER; 2.1% vs. 1.3–5.2% for LRR). These results could be attributed to the many benefits of MWA, including the higher intratumoral temperature, larger ablation zone, lower treatment time, and more complete tumor kill (7915). In addition, MWA may be less affected by the perfusion-mediated heat sink effect, which may treat tumors with a rich blood supply, and multiple antennae can be simultaneously applied to large tumors (635).
In the present meta-analysis, the summary incidences of major and minor complications associated with MWA were 1.8% (95% CI, 0.6–3.3%; I2 = 0.0%) and 17.5% (95% CI, 5.8–33.4%; I2 = 90.1%), respectively, which are comparable to the rates of 19.9% for cryotherapy and 19.0% for RFA reported in a recent meta-analytic summary involving cryotherapy and RFA (36). However, the incidence of major complications in MWA was lower than in RFA (0–5%) (3738394041) and cryotherapy (0–9%) (424344). Given the better technical and oncologic outcomes with a lower major complication rate, MWA appears to be an effective and safe treatment for malignant kidney tumors.
This study is subject to several limitations. First, both high study heterogeneity and a publication bias were noted in the incidence of minor complications. Although each study classified complications into major or minor types according to a pre-specified definition, the minor complications included a broad spectrum of events. Notably, due to the lack of an objective method of pain assessment, one study reported a high incidence of mild pain (13). In addition, the values of 3-year CSSR, TER for US, and LRR for CT showed moderate heterogeneity. Considering the fact that Gao et al. (12) only included RCCs adjacent to the renal sinus and the distance between the tumor and the collecting system was a predictive factor for complete renal ablation, the diversity of participants in Gao et al. (12), could have yielded different study results, i.e., the relatively low values of TER, high LRR, and low 3-year CSSR. Second, since we only analyzed published studies, there was a limitation in understanding the differences between studies in regard to censored subjects and their effect on the results. Third, as all included studies were retrospective, the study results might be limited.
In conclusion, MWA showed favorable technical and oncologic outcomes with a low incidence of major complications. Hence, we consider image-guided percutaneous MWA as a safe and effective treatment for malignant renal tumors.
References
1. Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999; 281:1628–1631. PMID: 10235157.

2. Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol. 2008; 179:75–79. discussion 79–80. PMID: 17997440.

3. Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009; 182:1271–1279. PMID: 19683266.

4. Horn JC, Patel RS, Kim E, Nowakowski FS, Lookstein RA, Fischman AM. Percutaneous microwave ablation of renal tumors using a gas-cooled 2.4-GHz probe: technique and initial results. J Vasc Interv Radiol. 2014; 25:448–453. PMID: 24581469.

5. Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010; 38:65–78. PMID: 21175404.

6. Laeseke PF, Lee FT Jr, Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009; 20:1224–1229. PMID: 19616970.

7. Bartoletti R, Cai T, Tosoratti N, Amabile C, Crisci A, Tinacci G, et al. In vivo microwave-induced porcine kidney thermoablation: results and perspectives from a pilot study of a new probe. BJU Int. 2010; 106:1817–1821. PMID: 20346045.

8. Carrafiello G, Dionigi G, Ierardi AM, Petrillo M, Fontana F, Floridi C, et al. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renal lesions Bosniak III or IV after 24 months follow up. Int J Surg. 2013; 11(Suppl 1):S30–S35. PMID: 24380547.

9. Moreland AJ, Ziemlewicz TJ, Best SL, Hinshaw JL, Lubner MG, Alexander ML, et al. High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol. 2014; 28:1046–1052. PMID: 24846329.

10. Yu J, Zhang G, Liang P, Yu XL, Cheng ZG, Han ZY, et al. Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radial nephrectomy for small renal cell carcinoma. Abdom Imaging. 2015; 40:3248–3256. PMID: 26288264.

11. Chen CN, Liang P, Yu J, Yu XL, Cheng ZG, Han ZY, et al. Contrast-enhanced ultrasound-guided percutaneous microwave ablation of renal cell carcinoma that is inconspicuous on conventional ultrasound. Int J Hyperthermia. 2016; 32:607–613. PMID: 27269816.

12. Gao Y, Liang P, Yu X, Yu J, Cheng Z, Han Z, et al. Microwave treatment of renal cell carcinoma adjacent to renal sinus. Eur J Radiol. 2016; 85:2083–2089. PMID: 27776662.

13. Li X, Yu J, Liang P, Yu X, Cheng Z, Han Z, et al. Combination therapy of three-dimensional (3D) visualisation operative treatment planning system and US-guided percutaneous microwave ablation in larger renal cell carcinomas (D ≥ 4 cm): preliminary results. Int J Hyperthermia. 2016; 1–7.
14. Wells SA, Wheeler KM, Mithqal A, Patel MS, Brace CL, Schenkman NS. Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single session treatment. Abdom Radiol (NY). 2016; 41:1203–1211. PMID: 27167230.

15. Chan P, Vélasco S, Vesselle G, Boucebci S, Herpe G, Debaene B, et al. Percutaneous microwave ablation of renal cancers under CT guidance: safety and efficacy with a 2-year follow-up. Clin Radiol. 2017; 72:786–792. PMID: 28545682.

16. Cheng Z, Yu X, Han Z, Liu F, Yu J, Liang P. Ultrasound-guided hydrodissection for assisting percutaneous microwave ablation of renal cell carcinomas adjacent to intestinal tracts: a preliminary clinical study. Int J Hyperthermia. 2017; 1–6.

17. Ierardi AM, Puliti A, Angileri SA, Petrillo M, Duka E, Floridi C, et al. Microwave ablation of malignant renal tumours: intermediate-term results and usefulness of RENAL and mRENAL scores for predicting outcomes and complications. Med Oncol. 2017; 34:97. PMID: 28421553.

18. Klapperich ME, Abel EJ, Ziemlewicz TJ, Best S, Lubner MG, Nakada SY, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology. 2017; 284:272–280. PMID: 28076721.

19. Mansilla AV, Bivins EE Jr, Contreras F, Hernandez MA, Kohler N, Pepe JW. CT-guided microwave ablation of 45 renal tumors: analysis of procedure complexity utilizing a percutaneous renal ablation complexity scoring system. J Vasc Interv Radiol. 2017; 28:222–229. PMID: 27988263.

20. Katsanos K, Mailli L, Krokidis M, McGrath A, Sabharwal T, Adam A. Systematic review and meta-analysis of thermal ablation versus surgical nephrectomy for small renal tumours. Cardiovasc Intervent Radiol. 2014; 37:427–437. PMID: 24482030.

21. Martin J, Athreya S. Meta-analysis of cryoablation versus microwave ablation for small renal masses: is there a difference in outcome? Diagn Interv Radiol. 2013; 19:501–507. PMID: 24084196.

22. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012. PMID: 10789670.
23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700. PMID: 19622552.

24. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339:b2535. PMID: 19622551.

25. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005; 235:728–739. PMID: 15845798.

26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.

27. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. statistical methods of meta-analysis. Korean J Radiol. 2015; 16:1188–1196. PMID: 26576107.

28. Campbell C, Lubner MG, Hinshaw JL, Muñoz del Rio A, Brace CL. Contrast media-doped hydrodissection during thermal ablation: optimizing contrast media concentration for improved visibility on CT images. AJR Am J Roentgenol. 2012; 199:677–682. PMID: 22915411.

29. Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer. 2010; 116:3135–3142. PMID: 20564644.
30. Lyrdal D, Andersson M, Hellström M, Sternal J, Lundstam S. Ultrasound-guided percutaneous radiofrequency ablation of small renal tumors: clinical results and radiological evolution during follow-up. Acta Radiol. 2010; 51:808–818. PMID: 20707665.

31. Zagoria RJ, Pettus JA, Rogers M, Werle DM, Childs D, Leyendecker JR. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011; 77:1393–1397. PMID: 21492910.

32. Rodriguez R, Cizman Z, Hong K, Koliatsos A, Georgiades C. Prospective analysis of the safety and efficacy of percutaneous cryoablation for pT1NxMx biopsy-proven renal cell carcinoma. Cardiovasc Intervent Radiol. 2011; 34:573–578. PMID: 20628879.

33. Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008; 113:2671–2680. PMID: 18816624.
34. Chalasani V, Martinez CH, Lim D, Abdelhady M, Chin JL. Surgical cryoablation as an option for small renal masses in patients who are not ideal partial nephrectomy candidates: intermediate-term outcomes. Can Urol Assoc J. 2010; 4:399–402. PMID: 21191499.

35. Carrafiello G, Laganà D, Mangini M, Fontana F, Dionigi G, Boni L, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008; 6(Suppl 1):S65–S69. PMID: 19186116.

36. El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int. 2012; 110:510–516. PMID: 22304329.

37. Ahrar K, Matin S, Wood CG, Wallace MJ, Gupta S, Madoff DC, et al. Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol. 2005; 16:679–688. PMID: 15872323.

38. Wah TM, Irving HC, Gregory W, Cartledge J, Joyce AD, Selby PJ. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int. 2014; 113:416–428. PMID: 24053769.

39. McDougal WS, Gervais DA, McGovern FJ, Mueller PR. Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol. 2005; 174:61–63. PMID: 15947578.

40. Weizer AZ, Raj GV, O'Connell M, Robertson CN, Nelson RC, Polascik TJ. Complications after percutaneous radiofrequency ablation of renal tumors. Urology. 2005; 66:1176–1180. PMID: 16360436.

41. Remzi M, Javadli E, Ozsoy M. Management of small renal masses: a review. World J Urol. 2010; 28:275–281. PMID: 20177900.

42. Okhunov Z, Shapiro EY, Moreira DM, Lipsky MJ, Hillelsohn J, Badani K, et al. R.E.N.A.L. nephrometry score accurately predicts complications following laparoscopic renal cryoablation. J Urol. 2012; 188:1796–1800. PMID: 22999696.

43. Kim EH, Tanagho YS, Bhayani SB, Saad NE, Benway BM, Figenshau RS. Percutaneous cryoablation of renal masses: Washington University experience of treating 129 tumours. BJU Int. 2013; 111:872–879. PMID: 23145500.

44. Schmit GD, Schenck LA, Thompson RH, Boorjian SA, Kurup AN, Weisbrod AJ, et al. Predicting renal cryoablation complications: new risk score based on tumor size and location and patient history. Radiology. 2014; 272:903–910. PMID: 24814178.

45. Guan W, Bai J, Liu J, Wang S, Zhuang Q, Ye Z, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012; 106:316–321. PMID: 22488716.

46. Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Mu MJ, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology. 2012; 263:900–908. PMID: 22495684.

47. Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Zhang X, et al. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology. 2014; 270:880–887. PMID: 24475805.

48. Li X, Liang P, Yu XL, Yu J, Cheng ZG, Han ZY, et al. Percutaneous microwave ablation for renal cell carcinoma: evaluation of therapeutic effect with contrast-enhanced ultrasound. J Interv Radiol (China). 2014; 23:688–692.
49. Han ZY, Liang P, Yu XL, Cheng ZG, Liu FY, Yu J. Ultrasound-guided percutaneous microwave ablation of sporadic renal angiomyolipoma: preliminary results. Acta Radiol. 2015; 56:56–62. PMID: 24526757.

50. Cristescu M, Abel EJ, Wells S, Ziemlewicz TJ, Hedican SP, Lubner MG, et al. Percutaneous microwave ablation of renal angiomyolipomas. Cardiovasc Intervent Radiol. 2016; 39:433–440. PMID: 26390876.

51. Mu MJ, Yu J, Liang P, Yu XL, Han ZY, Cheng ZG, et al. [Long-term effects of ultrasound-guided microwave ablation in the treatment of small renal cell carcinoma]. Nan Fang Yi Ke Da Xue Xue Bao. 2016; 36:622–627. PMID: 27222174.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3348/kjr.2018.19.5.938.
Supplementary Table 1
Specific Reasons for Excluding Seven Articles after Full-Text Review
Supplementary Fig. 2
Forest plot (left) and funnel plot (right) for local tumor recurrence.
Supplementary Fig. 3
Funnel plots evaluating publication bias for technical outcomes, oncologic outcomes, and complications.
Fig. 1
Flow diagram outlining article selection process.
Article may have been excluded for multiple reasons, but only one major reason per article is presented.
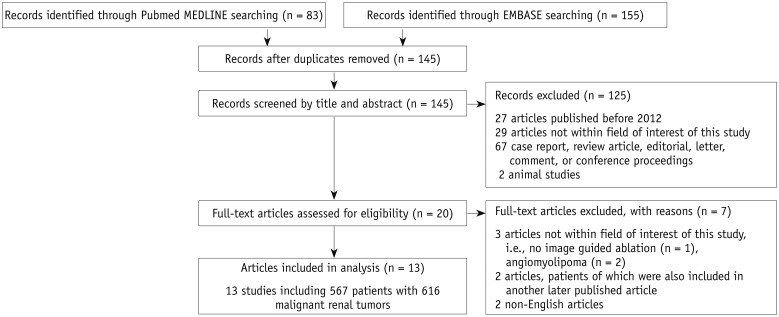
Fig. 2
Meta-analytic summary of technical outcomes.
Forest plots for technical success rate (left) and technical efficacy rate (right). Study ID provides first author's name and year of publication. Diamond marker indicates meta-analytic summary estimate. CI = confidence interval
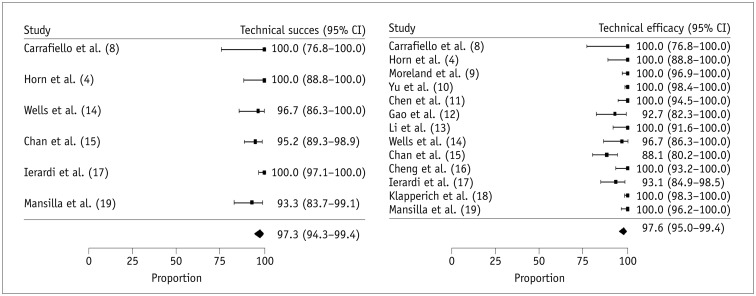
Fig. 3
Meta-analytic summary of oncologic outcomes.
A. Forest plot for cancer-specific survival rate. B. Forest plot for overall survival rate.
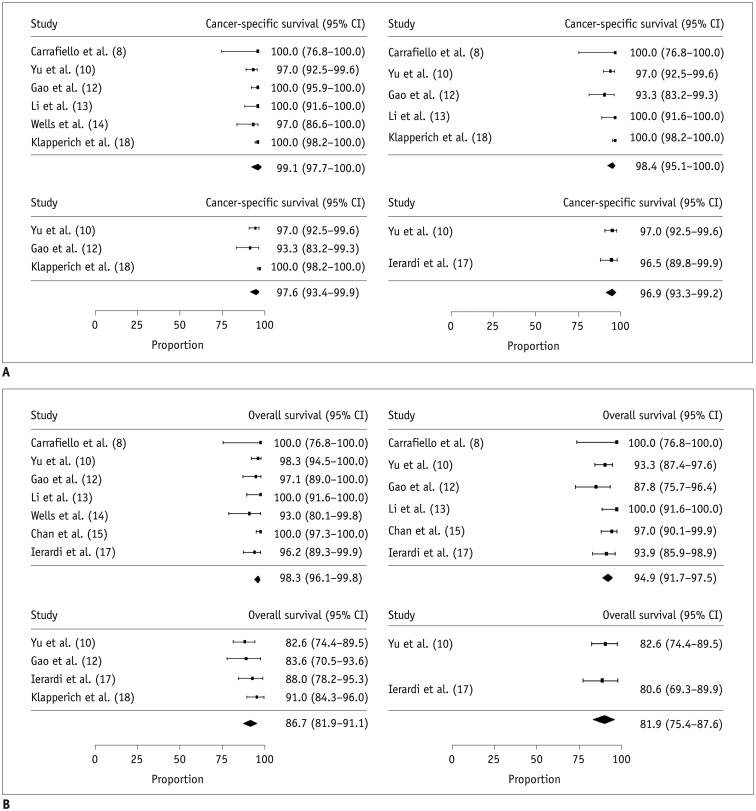
Fig. 4
Meta-analytic summary of complications.
Forest plot for major complications (left) and minor complications (right).
Table 1
Characteristics of Articles Included
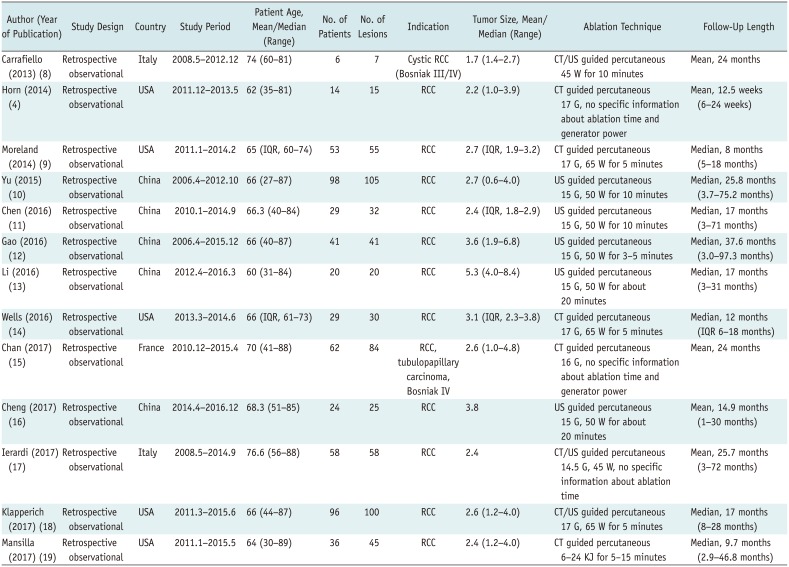
| Author (Year of Publication) | Study Design | Country | Study Period | Patient Age, Mean/Median (Range) | No. of Patients | No. of Lesions | Indication | Tumor Size, Mean/Median (Range) | Ablation Technique | Follow-Up Length |
|---|---|---|---|---|---|---|---|---|---|---|
| Carrafiello (2013) (8) | Retrospective observational | Italy | 2008.5–2012.12 | 74 (60–81) | 6 | 7 | Cystic RCC (Bosniak III/IV) | 1.7 (1.4–2.7) | CT/US guided percutaneous 45 W for 10 minutes | Mean, 24 months |
| Horn (2014) (4) | Retrospective observational | USA | 2011.12–2013.5 | 62 (35–81) | 14 | 15 | RCC | 2.2 (1.0–3.9) | CT guided percutaneous 17 G, no specific information about ablation time and generator power | Mean, 12.5 weeks (6–24 weeks) |
| Moreland (2014) (9) | Retrospective observational | USA | 2011.1–2014.2 | 65 (IQR, 60–74) | 53 | 55 | RCC | 2.7 (IQR, 1.9–3.2) | CT guided percutaneous 17 G, 65 W for 5 minutes | Median, 8 months (5–18 months) |
| Yu (2015) (10) | Retrospective observational | China | 2006.4–2012.10 | 66 (27–87) | 98 | 105 | RCC | 2.7 (0.6–4.0) | US guided percutaneous 15 G, 50 W for 10 minutes | Median, 25.8 months (3.7–75.2 months) |
| Chen (2016) (11) | Retrospective observational | China | 2010.1–2014.9 | 66.3 (40–84) | 29 | 32 | RCC | 2.4 (IQR, 1.8–2.9) | US guided percutaneous 15 G, 50 W for 10 minutes | Median, 17 months (3–71 months) |
| Gao (2016) (12) | Retrospective observational | China | 2006.4–2015.12 | 66 (40–87) | 41 | 41 | RCC | 3.6 (1.9–6.8) | US guided percutaneous 15 G, 50 W for 3–5 minutes | Median, 37.6 months (3.0–97.3 months) |
| Li (2016) (13) | Retrospective observational | China | 2012.4–2016.3 | 60 (31–84) | 20 | 20 | RCC | 5.3 (4.0–8.4) | US guided percutaneous 15 G, 50 W for about 20 minutes | Median, 17 months (3–31 months) |
| Wells (2016) (14) | Retrospective observational | USA | 2013.3–2014.6 | 66 (IQR, 61–73) | 29 | 30 | RCC | 3.1 (IQR, 2.3–3.8) | CT guided percutaneous 17 G, 65 W for 5 minutes | Median, 12 months (IQR 6–18 months) |
| Chan (2017) (15) | Retrospective observational | France | 2010.12–2015.4 | 70 (41–88) | 62 | 84 | RCC, tubulopapillary carcinoma, Bosniak IV | 2.6 (1.0–4.8) | CT guided percutaneous 16 G, no specific information about ablation time and generator power | Mean, 24 months |
| Cheng (2017) (16) | Retrospective observational | China | 2014.4–2016.12 | 68.3 (51–85) | 24 | 25 | RCC | 3.8 | US guided percutaneous 15 G, 50 W for about 20 minutes | Mean, 14.9 months (1–30 months) |
| Ierardi (2017) (17) | Retrospective observational | Italy | 2008.5–2014.9 | 76.6 (56–88) | 58 | 58 | RCC | 2.4 | CT/US guided percutaneous 14.5 G, 45 W, no specific information about ablation time | Mean, 25.7 months (3–72 months) |
| Klapperich (2017) (18) | Retrospective observational | USA | 2011.3–2015.6 | 66 (44–87) | 96 | 100 | RCC | 2.6 (1.2–4.0) | CT/US guided percutaneous 17 G, 65 W for 5 minutes | Median, 17 months (8–28 months) |
| Mansilla (2017) (19) | Retrospective observational | USA | 2011.1–2015.5 | 64 (30–89) | 36 | 45 | RCC | 2.4 (1.2–4.0) | CT guided percutaneous 6–24 KJ for 5–15 minutes | Median, 9.7 months (2.9–46.8 months) |
Table 2
Oncologic Outcomes Reported in Individual Studies at 1-, 2-, 3-, and 5-Year Follow-Up Times
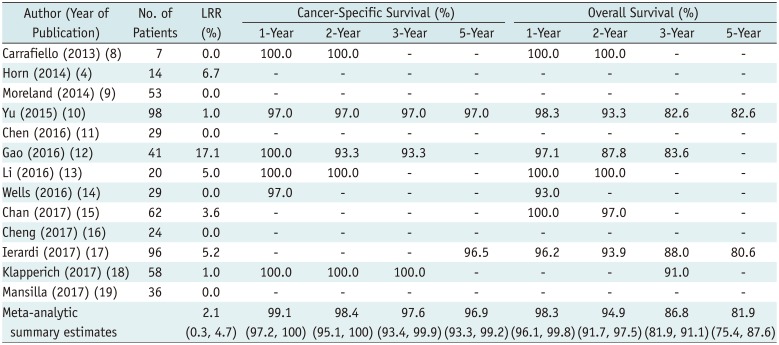
Table 3
Summary of Complications after MWA
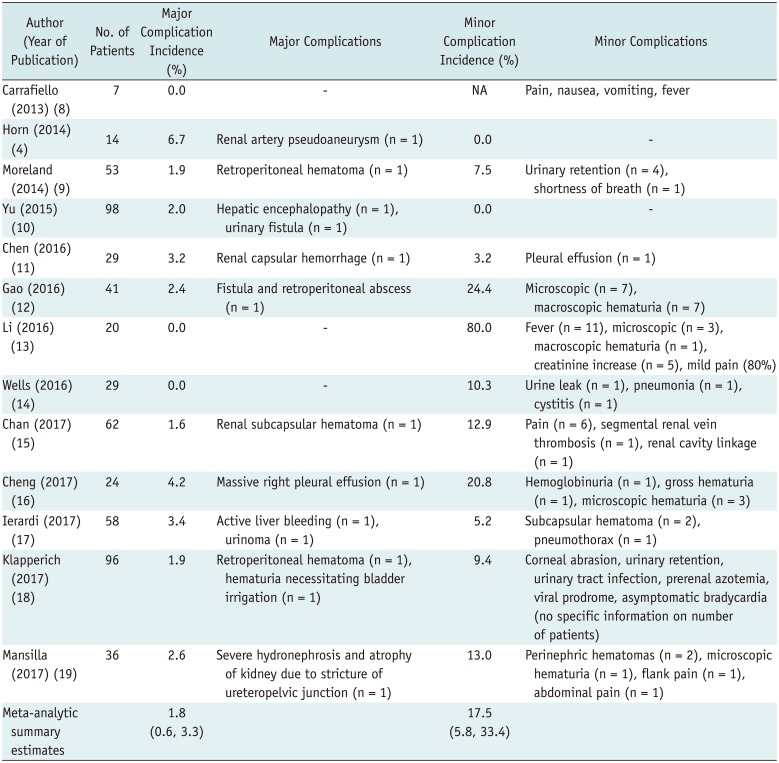
Table 4
Subgroup Analysis according to Type of Image Guidance




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download