1. Huang SW, Lin JW, Wang WT, Wu CW, Liou TH, Lin HW. Hyperthyroidism is a risk factor for developing adhesive capsulitis of the shoulder: a nationwide longitudinal population-based study. Sci Rep. 2014; 4:4183. PMID:
24567049.

2. Neviaser JS. Adhesive capsulitis and the stiff and painful shoulder. Orthop Clin North Am. 1980; 11:327–331. PMID:
7001312.

3. Neviaser AS, Hannafin JA. Adhesive capsulitis: a review of current treatment. Am J Sports Med. 2010; 38:2346–2356. PMID:
20110457.
4. Emig EW, Schweitzer ME, Karasick D, Lubowitz J. Adhesive capsulitis of the shoulder: MR diagnosis. AJR Am J Roentgenol. 1995; 164:1457–1459. PMID:
7754892.

5. Mengiardi B, Pfirrmann CW, Gerber C, Hodler J, Zanetti M. Frozen shoulder: MR arthrographic findings. Radiology. 2004; 233:486–492. PMID:
15358849.

6. Gondim Teixeira PA, Balaj C, Chanson A, Lecocq S, Louis M, Blum A. Adhesive capsulitis of the shoulder: value of inferior glenohumeral ligament signal changes on T2-weighted fat-saturated images. AJR Am J Roentgenol. 2012; 198:W589–W596. PMID:
22623575.
7. Connell D, Padmanabhan R, Buchbinder R. Adhesive capsulitis: role of MR imaging in differential diagnosis. Eur Radiol. 2002; 12:2100–2106. PMID:
12136330.

8. MacDougall JD, Elder GC, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibres. Eur J Appl Physiol Occup Physiol. 1980; 43:25–34. PMID:
7371625.

9. Lin HC, Li JS, Lo SF, Shih YF, Lo CY, Chen SY. Isokinetic characteristics of shoulder rotators in patients with adhesive capsulitis. J Rehabil Med. 2009; 41:563–568. PMID:
19543668.

10. Rawat P, Eapen C, Seema KP. Effect of rotator cuff strengthening as an adjunct to standard care in subjects with adhesive capsulitis: A randomized controlled trial. J Hand Ther. 2017; 30:235–241.e8. PMID:
27884497.

11. Agten CA, Rosskopf AB, Gerber C, Pfirrmann CW. Quantification of early fatty infiltration of the rotator cuff muscles: comparison of multi-echo Dixon with single-voxel MR spectroscopy. Eur Radiol. 2016; 26:3719–3727. PMID:
26679183.

12. Fischer MA, Nanz D, Shimakawa A, Schirmer T, Guggenberger R, Chhabra A, et al. Quantification of muscle fat in patients with low back pain: comparison of multi-echo MR imaging with single-voxel MR spectroscopy. Radiology. 2013; 266:555–563. PMID:
23143025.

13. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990; 64–74.

14. Hannafin JA, Chiaia TA. Adhesive capsulitis. A treatment approach. Clin Orthop Relat Res. 2000; 95–109. PMID:
10738419.
15. Zhong X, Nickel MD, Kannengiesser SA, Dale BM, Kiefer B, Bashir MR. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med. 2014; 72:1353–1365. PMID:
24323332.

16. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004; 22:1004–1007. PMID:
15304272.

17. Park S, Lee DH, Yoon SH, Lee HY, Kwack KS. Evaluation of adhesive capsulitis of the shoulder with fat-suppressed T2-weighted MRI: Association between clinical features and MRI findings. AJR Am J Roentgenol. 2016; 207:135–141. PMID:
27070051.

18. Ackland DC, Pak P, Richardson M, Pandy MG. Moment arms of the muscles crossing the anatomical shoulder. J Anat. 2008; 213:383–390. PMID:
18691376.

19. Nardo L, Karampinos DC, Lansdown DA, Carballido-Gamio J, Lee S, Maroldi R, et al. Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. J Magn Reson Imaging. 2014; 39:1178–1185. PMID:
24115490.

20. Ahn KS, Kang CH, Oh YW, Jeong WK. Correlation between magnetic resonance imaging and clinical impairment in patients with adhesive capsulitis. Skeletal Radiol. 2012; 41:1301–1308. PMID:
22430562.

21. Walch G, Boulahia A, Calderone S, Robinson AH. The ‘dropping’ and ‘hornblower's’ signs in evaluation of rotator-cuff tears. J Bone Joint Surg Br. 1998; 80:624–628. PMID:
9699824.

22. Jung JY, Jee WH, Chun HJ, Kim YS, Chung YG, Kim JM. Adhesive capsulitis of the shoulder: evaluation with MR arthrography. Eur Radiol. 2006; 16:791–796. PMID:
16228212.

23. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994; 78–83. PMID:
8020238.
24. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999; 8:599–605. PMID:
10633896.

25. Tae SK, Oh JH, Kim SH, Chung SW, Yang JY, Back YW. Evaluation of fatty degeneration of the supraspinatus muscle using a new measuring tool and its correlation between multidetector computed tomography and magnetic resonance imaging. Am J Sports Med. 2011; 39:599–606. PMID:
21148143.

26. Fox PT. Physiological ROI definition by image subtraction. J Cereb Blood Flow Metab. 1991; 11:A79–A82. PMID:
1997492.

27. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010; 468:1558–1564. PMID:
19347412.

28. Slabaugh MA, Friel NA, Karas V, Romeo AA, Verma NN, Cole BJ. Interobserver and intraobserver reliability of the Goutallier classification using magnetic resonance imaging: proposal of a simplified classification system to increase reliability. Am J Sports Med. 2012; 40:1728–1734. PMID:
22753846.
29. Lesage P, Maynou C, Elhage R, Boutry N, Hérent S, Mestdagh H. [Reproducibility of CT scan evaluation of muscular fatty degeneration. Intra- and interobserver analysis of 56 shoulders presenting with a ruptured rotator cuff muscles]. Rev Chir Orthop Reparatrice Appar Mot. 2002; 88:359–364. PMID:
12124535.
30. Yoo YH, Kim HS, Lee YH, Yoon CS, Paek MY, Yoo H, et al. Comparison of multi-echo Dixon methods with volume interpolated breath-hold gradient echo magnetic resonance imaging in fat-signal fraction quantification of paravertebral muscle. Korean J Radiol. 2015; 16:1086–1095. PMID:
26357503.

31. Reeder SB, Robson PM, Yu H, Shimakawa A, Hines CD, McKenzie CA, et al. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009; 29:1332–1339. PMID:
19472390.

32. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001; 10:199–203. PMID:
11408898.

33. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don't lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006; 88:116–121. PMID:
16551396.

34. Needell SD, Zlatkin MB, Sher JS, Murphy BJ, Uribe JW. MR imaging of the rotator cuff: peritendinous and bone abnormalities in an asymptomatic population. AJR Am J Roentgenol. 1996; 166:863–867. PMID:
8610564.

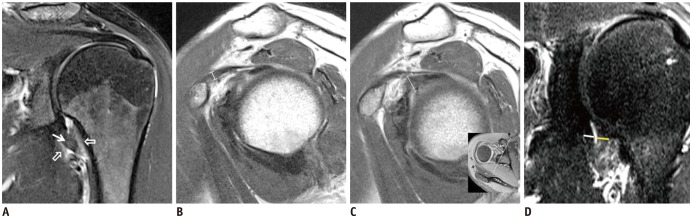
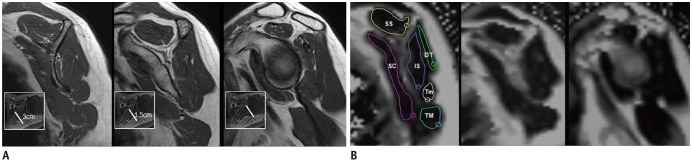
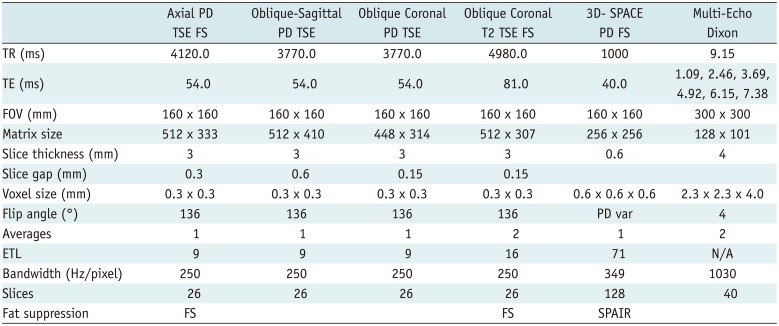
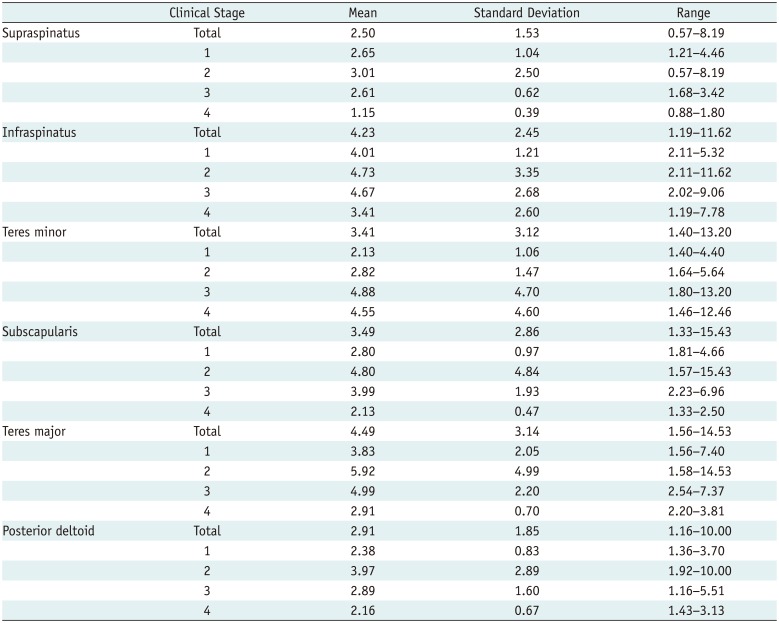




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download