1. Matsumura T, Hayakawa M, Shimada F, Yabuki M, Dohanish S, Palkowitsch P, et al. Safety of gadopentetate dimeglumine after 120 million administrations over 25 years of clinical use. Magn Reson Med Sci. 2013; 12:297–304. PMID:
24172794.

2. Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol. 1996; 167:847–849. PMID:
8819369.

3. Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000; 12:205–213. PMID:
10931582.

4. Runge VM. Safety of magnetic resonance contrast media. Top Magn Reson Imaging. 2001; 12:309–314. PMID:
11687717.

5. Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006; 17:2359–2362. PMID:
16885403.

6. Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006; 21:1104–1108. PMID:
16431890.

7. Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008; 18:2164–2173. PMID:
18545998.

8. Perez-Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment--report of 33 cases. Radiology. 2009; 250:371–377. PMID:
19188312.

9. Thomsen HS. Nephrogenic systemic fibrosis: history and epidemiology. Radiol Clin North Am. 2009; 47:827–831. viPMID:
19744597.

10. ACR Committee on Drugs and Contrast Media. Nephrogenic Systemic Fibrosis. ACR Committee on Drugs and Contrast Media. ACR manual on contrast media, version 10.3. Reston, VA: American College of Radiology;2017. p. 81–89.
11. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014; 270:834–841. PMID:
24475844.

12. Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014; 49:685–690. PMID:
24872007.

13. Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM. Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol. 2015; 36:1859–1865. PMID:
26294649.

14. Cao Y, Huang DQ, Shih G, Prince MR. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol. 2016; 206:414–419. PMID:
26700156.

15. Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR Images: association with linear versus macrocyclic gadolinium chelate administration. Radiology. 2015; 275:803–809. PMID:
25633504.

16. Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015; 275:783–791. PMID:
25848905.

17. Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, et al. High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology. 2015; 276:836–844. PMID:
26079490.

18. Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, et al. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol. 2015; 50:743–748. PMID:
26352749.

19. Hu HH, Pokorney A, Towbin RB, Miller JH. Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol. 2016; 46:1590–1598. PMID:
27282825.

20. Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, et al. Intraindividual analysis of signal intensity changes in the dentate nucleus after consecutive serial applications of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol. 2016; 51:683–690. PMID:
27495187.

21. Ramalho J, Ramalho M, AlObaidy M, Nunes RH, Castillo M, Semelka RC. T1 signal-intensity increase in the dentate nucleus after multiple exposures to gadodiamide: intraindividual comparison between 2 commonly used sequences. AJNR Am J Neuroradiol. 2016; 37:1427–1431. PMID:
27032972.

22. Ramalho J, Semelka RC, AlObaidy M, Ramalho M, Nunes RH, Castillo M. Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol. 2016; 26:4080–4088. PMID:
26911888.

23. Roberts DR, Chatterjee AR, Yazdani M, Marebwa B, Brown T, Collins H, et al. Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol. 2016; 37:2340–2347. PMID:
27469211.

24. Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev. 2016; 38:331–336. PMID:
26345358.

25. Tanaka M, Nakahara K, Kinoshita M. Increased signal intensity in the dentate nucleus of patients with multiple sclerosis in comparison with neuromyelitis optica spectrum disorder after multiple doses of gadolinium contrast. Eur Neurol. 2016; 75:195–198. PMID:
27054693.

26. Flood TF, Stence NV, Maloney JA, Mirsky DM. Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology. 2017; 282:222–228. PMID:
27467467.

27. Ichikawa S, Motosugi U, Omiya Y, Onishi H. Contrast agent-induced high signal intensity in dentate nucleus on unenhanced T1-weighted images: comparison of gadodiamide and gadoxetic acid. Invest Radiol. 2017; 52:389–395. PMID:
28195932.
28. Kuno H, Jara H, Buch K, Qureshi MM, Chapman MN, Sakai O. Global and regional brain assessment with quantitative MR imaging in patients with prior exposure to linear gadolinium-based contrast agents. Radiology. 2017; 283:195–204. PMID:
27797676.

29. Öner AY, Barutcu B, Aykol S¸, Tali ET. Intrathecal contrast-enhanced magnetic resonance imaging-related brain signal changes: residual gadolinium deposition? Invest Radiol. 2017; 52:195–197. PMID:
27755154.
30. Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR. Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology. 2017; 282:516–525. PMID:
27513848.

31. Drug Safety Communications. FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue. U.S. Food & Drug Administration;Accessed May 26, 2018. Retrieved from
https://www.fda.gov/downloads/Drugs/DrugSafety/UCM559654.pdf. Published July 27, 2015.
34. Pharmaceuticals and Medical Devices Agency. Report on the investigation results. Pharmaceuticals and Medical Devices Agency;Accessed May 26, 2018. Retrieved from
http://www.pmda.go.jp/files/000221379.pdf. Published November 11, 2017.
35. Advance notice on mandatory revision of the precautions section in the package insert of gadolinium agent. Ministry of Food and Drug Safety Web site. Accessed May 26, 2018.
http://www.mfds.go.kr/brd/m_74/view.do?seq=40761. Published February 27, 2018.
37. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016; 207:229–233. PMID:
27224028.

38. Pałasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000; 47:1107–1114. PMID:
11996100.

39. Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008; 66:175–179. PMID:
18343072.

40. Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009; 30:1249–1258. PMID:
19938037.
41. Tweedle MF, Hagan JJ, Kumar K, Mantha S, Chang CA. Reaction of gadolinium chelates with endogenously available ions. Magn Reson Imaging. 1991; 9:409–415. PMID:
1881260.

42. Mann JS. Stability of gadolinium complexes in vitro and in vivo. J Comput Assist Tomogr. 1993; 17(Suppl 1):S19–S23. PMID:
8486827.

43. Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37℃. Invest Radiol. 2008; 43:817–828. PMID:
19002053.
44. Port M, Idée JM, Medina C, Robic C, Sabatou M, Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008; 21:469–490. PMID:
18344005.

45. Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology. 2009; 251:503–510. PMID:
19401576.

46. Prayer D, Grois N, Prosch H, Gadner H, Barkovich AJ. MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis. AJNR Am J Neuroradiol. 2004; 25:880–891. PMID:
15140741.
47. Kasahara S, Miki Y, Kanagaki M, Yamamoto A, Mori N, Sawada T, et al. Hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with a history of brain irradiation. Radiology. 2011; 258:222–228. PMID:
21045180.

48. Lai PH, Chen C, Liang HL, Pan HB. Hyperintense basal ganglia on T1-weighted MR imaging. AJR Am J Roentgenol. 1999; 172:1109–1115. PMID:
10587157.

49. Oikonomou A, Chatzistefanou A, Zezos P, Mintzopoulou P, Vadikolias K, Prassopoulos P. Basal ganglia hyperintensity on T1-weighted MRI in Rendu-Osler-Weber disease. J Magn Reson Imaging. 2012; 35:426–430. PMID:
22127848.

50. da Silva CJ, da Rocha AJ, Jeronymo S, Mendes MF, Milani FT, Maia AC Jr, et al. A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: manganism symptoms and T1 hyperintense changes in the basal ganglia. AJNR Am J Neuroradiol. 2007; 28:1474–1479. PMID:
17846194.

51. Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, et al. Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol. 2015; 50:470–472. PMID:
25756685.

52. Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol. 2015; 50:805–810. PMID:
26523910.
53. Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol. 2016; 26:807–815. PMID:
26105022.

54. Khant ZA, Hirai T, Kadota Y, Masuda R, Yano T, Azuma M, et al. T1 shortening in the cerebral cortex after multiple administrations of gadolinium-based contrast agents. Magn Reson Med Sci. 2017; 16:84–86. PMID:
27725576.
55. McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015; 275:772–782. PMID:
25742194.

56. McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Paolini MA, Murray DL, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017; 285:546–554. PMID:
28653860.
57. Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015; 276:228–232. PMID:
25942417.

58. Lohrke J, Frisk AL, Frenzel T, Schöckel L, Rosenbruch M, Jost G, et al. Histology and gadolinium distribution in the rodent brain after the administration of cumulative high doses of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol. 2017; 52:324–333. PMID:
28323657.

59. Smith AP, Marino M, Roberts J, Crowder JM, Castle J, Lowery L, et al. Clearance of gadolinium from the brain with no pathologic effect after repeated administration of gadodiamide in healthy rats: an analytical and histologic study. Radiology. 2017; 282:743–751. PMID:
27673510.
60. McDonald JS, McDonald RJ, Jentoft ME, Paolini MA, Murray DL, Kallmes DF, et al. Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr. 2017; 171:705–707. PMID:
28531274.
61. Gianolio E, Bardini P, Arena F, Stefania R, Di Gregorio E, Iani R, et al. Gadolinium retention in the rat brain: assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology. 2017; 285:839–849. PMID:
28873047.
62. Frenzel T, Apte C, Jost G, Schöckel L, Lohrke J, Pietsch H. Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: comparative study in rats. Invest Radiol. 2017; 52:396–404. PMID:
28125438.
63. Greenberg SA. Zinc transmetallation and gadolinium retention after MR imaging: case report. Radiology. 2010; 257:670–673. PMID:
20829541.

64. Swaminathan S. Gadolinium toxicity: iron and ferroportin as central targets. Magn Reson Imaging. 2016; 34:1373–1376. PMID:
27580520.

65. Kanda T, Nakai Y, Oba H, Toyoda K, Kitajima K, Furui S. Gadolinium deposition in the brain. Magn Reson Imaging. 2016; 34:1346–1350. PMID:
27613998.

66. Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idée JM, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol. 2015; 50:473–480. PMID:
26107651.
67. Robert P, Violas X, Grand S, Lehericy S, Idée JM, Ballet S, et al. Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol. 2016; 51:73–82. PMID:
26606549.

68. Kartamihardja AA, Nakajima T, Kameo S, Koyama H, Tsushima Y. Distribution and clearance of retained gadolinium in the brain: differences between linear and macrocyclic gadolinium based contrast agents in a mouse model. Br J Radiol. 2016; 89:20160509. PMID:
27459250.

69. Kartamihardja AA, Nakajima T, Kameo S, Koyama H, Tsushima Y. Impact of impaired renal function on gadolinium retention after administration of gadolinium-based contrast agents in a mouse model. Invest Radiol. 2016; 51:655–660. PMID:
27299580.

70. Swaminathan S, Horn TD, Pellowski D, Abul-Ezz S, Bornhorst JA, Viswamitra S, et al. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med. 2007; 357:720–722. PMID:
17699829.

71. Swaminathan S, Shah SV. New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol. 2007; 18:2636–2643. PMID:
17855637.

72. Kanda T, Oba H, Toyoda K, Kitajima K, Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol. 2016; 34:3–9. PMID:
26608061.

73. Valdés Hernández Mdel C, Maconick LC, Tan EM, Wardlaw JM. Identification of mineral deposits in the brain on radiological images: a systematic review. Eur Radiol. 2012; 22:2371–2381. PMID:
22688125.

74. Bressler JP, Olivi L, Cheong JH, Kim Y, Maerten A, Bannon D. Metal transporters in intestine and brain: their involvement in metal-associated neurotoxicities. Hum Exp Toxicol. 2007; 26:221–229. PMID:
17439925.

75. Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H. Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Invest Radiol. 2016; 51:83–89. PMID:
26606548.
76. Mamourian AC, Hoopes PJ, Lewis LD. Visualization of intravenously administered contrast material in the CSF on fluid-attenuated inversion-recovery MR images: an in vitro and animal-model investigation. AJNR Am J Neuroradiol. 2000; 21:105–111. PMID:
10669233.
77. Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013; 123:1299–1309. PMID:
23434588.

78. Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open. 2015; 4:2058460115609635. PMID:
26634147.

79. Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn Reson Med Sci. 2017; 16:61–65. PMID:
27430361.

80. Eisele P, Alonso A, Szabo K, Ebert A, Ong M, Schoenberg SO, et al. Lack of increased signal intensity in the dentate nucleus after repeated administration of a macrocyclic contrast agent in multiple sclerosis: an observational study. Medicine (Baltimore). 2016; 95:e4624. PMID:
27684794.
81. Kromrey ML, Liedtke KR, Ittermann T, Langner S, Kirsch M, Weitschies W, et al. Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol. 2017; 27:772–777. PMID:
27221561.
82. Lee JY, Park JE, Kim HS, Kim SO, Oh JY, Shim WH, et al. Up to 52 administrations of macrocyclic ionic MR contrast agent are not associated with intracranial gadolinium deposition: multifactorial analysis in 385 patients. PLoS One. 2017; 12:e0183916. PMID:
28859167.

83. Yoo RE, Sohn CH, Kang KM, Yun TJ, Choi SH, Kim JH, et al. Evaluation of gadolinium retention after serial administrations of a macrocyclic gadolinium-based contrast agent (gadobutrol): a single-institution experience with 189 patients. Invest Radiol. 2018; 53:20–25. PMID:
28742734.
84. Schlemm L, Chien C, Bellmann-Strobl J, Dörr J, Wuerfel J, Brandt AU, et al. Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler. 2017; 23:963–972. PMID:
27679460.

85. Ryu YJ, Choi YH, Cheon JE, Lee WJ, Park S, Park JE, et al. Pediatric brain: gadolinium deposition indentate nucleus and globus pallidus on unenhanced T1-weighted images is dependent on the type of contrast agent. Invest Radiol. 2018; 53:246–255. PMID:
29300210.
86. Kahn J, Posch H, Steffen IG, Geisel D, Bauknecht C, Liebig T, et al. Is there long-term signal intensity increase in the central nervous system on T1-weighted images after MR imaging with the hepatospecific contrast agent gadoxetic acid? A cross-sectional study in 91 patients. Radiology. 2017; 282:708–716. PMID:
28076722.

87. Conte G, Preda L, Cocorocchio E, Raimondi S, Giannitto C, Minotti M, et al. Signal intensity change on unenhanced T1-weighted images in dentate nucleus and globus pallidus after multiple administrations of gadoxetate disodium: an intraindividual comparative study. Eur Radiol. 2017; 27:4372–4378. PMID:
28357495.

88. Kang KM, Choi SH, Hwang M, Yun TJ, Kim JH, Sohn CH. T1 shortening in the globus pallidus after multiple administrations of gadobutrol: assessment with a multidynamic multiecho sequence. Radiology. 2018; 287:258–266. PMID:
29091750.

89. Rossi Espagnet MC, Bernardi B, Pasquini L, Figà-Talamanca L, Tomà P, Napolitano A. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol. 2017; 47:1345–1352. PMID:
28526896.

90. Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016; 51:447–453. PMID:
26863577.
91. McDonald RJ, McDonald JS, Dai D, Schroeder D, Jentoft ME, Murray DL, et al. Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology. 2017; 285:536–545. PMID:
28640692.
92. Rasschaert M, Idée JM, Robert P, Fretellier N, Vives V, Violas X, et al. Moderate renal failure accentuates T1 signal enhancement in the deep cerebellar nuclei of gadodiamide-treated rats. Invest Radiol. 2017; 52:255–264. PMID:
28067754.

93. Boyken J, Frenzel T, Lohrke J, Jost G, Pietsch H. Gadolinium accumulation in the deep cerebellar nuclei and globus pallidus after exposure to linear but not macrocyclic gadolinium-based contrast agents in a retrospective pig study with high similarity to clinical conditions. Invest Radiol. 2018; 53:278–285. PMID:
29319556.

94. Bussi S, Coppo A, Botteron C, Fraimbault V, Fanizzi A, De Laurentiis E, et al. Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging. 2018; 47:746–752. PMID:
28730643.

95. Cao Y, Zhang Y, Shih G, Zhang Y, Bohmart A, Hecht EM, et al. Effect of renal function on gadolinium-related signal increases on unenhanced T1-weighted brain magnetic resonance imaging. Invest Radiol. 2016; 51:677–682. PMID:
27272543.

96. Tibussek D, Rademacher C, Caspers J, Turowski B, Schaper J, Antoch G, et al. Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology. 2017; 285:223–230. PMID:
28640695.
97. Tedeschi E, Palma G, Canna A, Cocozza S, Russo C, Borrelli P, et al. In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol. 2016; 26:4577–4584. PMID:
26905870.

98. Hinoda T, Fushimi Y, Okada T, Arakawa Y, Liu C, Yamamoto A, et al. Quantitative assessment of gadolinium deposition in dentate nucleus using quantitative susceptibility mapping. J Magn Reson Imaging. 2017; 45:1352–1358. PMID:
27664936.

99. Welk B, McArthur E, Morrow SA, MacDonald P, Hayward J, Leung A, et al. Association between gadolinium contrast exposure and the risk of parkinsonism. JAMA. 2016; 316:96–98. PMID:
27380348.

100. Bauer K, Lathrum A, Raslan O, Kelly PV, Zhou Y, Hewing D, et al. Do gadolinium-based contrast agents affect
18F-FDG PET/CT uptake in the dentate nucleus and the globus pallidus? A pilot study. J Nucl Med Technol. 2017; 45:30–33. PMID:
27834725.
101. Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, et al. Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn Reson Imaging. 2016; 34:1383–1390. PMID:
27530966.

102. Muldoon LL, Neuwelt EA. Dose-dependent neurotoxicity (seizures) due to deposition of gadolinium-based contrast agents in the central nervous system. Radiology. 2015; 277:925–926.

103. Roman-Goldstein SM, Barnett PA, McCormick CI, Ball MJ, Ramsey F, Neuwelt EA. Effects of gadopentetate dimeglumine administration after osmotic blood-brain barrier disruption: toxicity and MR imaging findings. AJNR Am J Neuroradiol. 1991; 12:885–890. PMID:
1950917.
104. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018; 48:330–340. PMID:
29437269.

105. Tweedle MF. Science to practice: will gadolinium chelates be replaced by iron chelates in MR imaging? Radiology. 2018; 286:409–411. PMID:
29356647.

106. Chan KW, McMahon MT, Kato Y, Liu G, Bulte JW, Bhujwalla ZM, et al. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med. 2012; 68:1764–1773. PMID:
23074027.
107. Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014; 15:554–577. PMID:
25246817.

108. Ramalho J, Ramalho M. Gadolinium deposition and chronic toxicity. Magn Reson Imaging Clin N Am. 2017; 25:765–778. PMID:
28964466.

109. ACR Committee on Drugs and Contrast Media. Appendix A - Contrast media specifications. ACR manual on contrast media, version 10.3. Reston, VA: American College of Radiology;2017. p. 123–125.
110. Huckle JE, Altun E, Jay M, Semelka RC. Gadolinium deposition in humans: when did we learn that gadolinium was deposited in vivo? Invest Radiol. 2016; 51:236–240. PMID:
26588463.
111. Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol. 2016; 37:1192–1198. PMID:
26659341.

112. Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017; 16:564–570. PMID:
28653648.

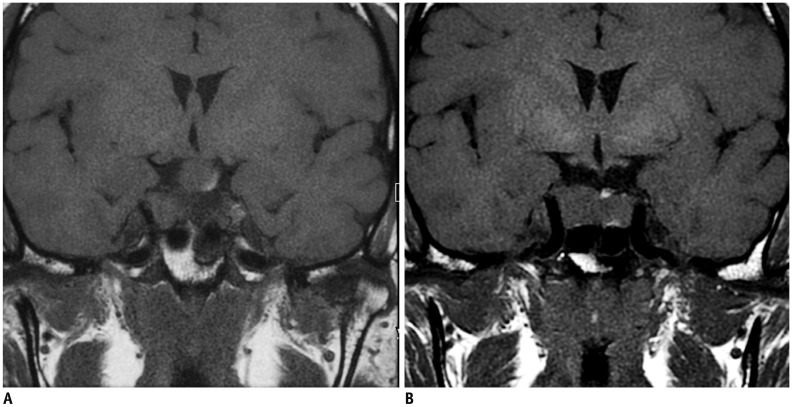
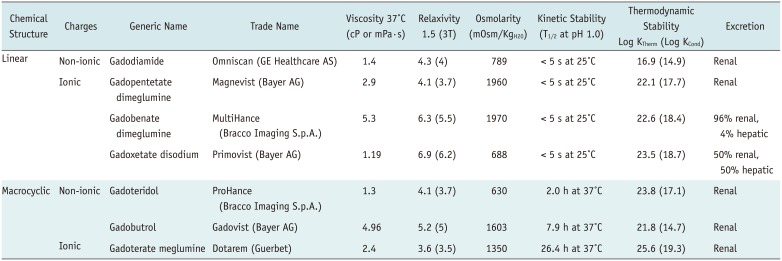
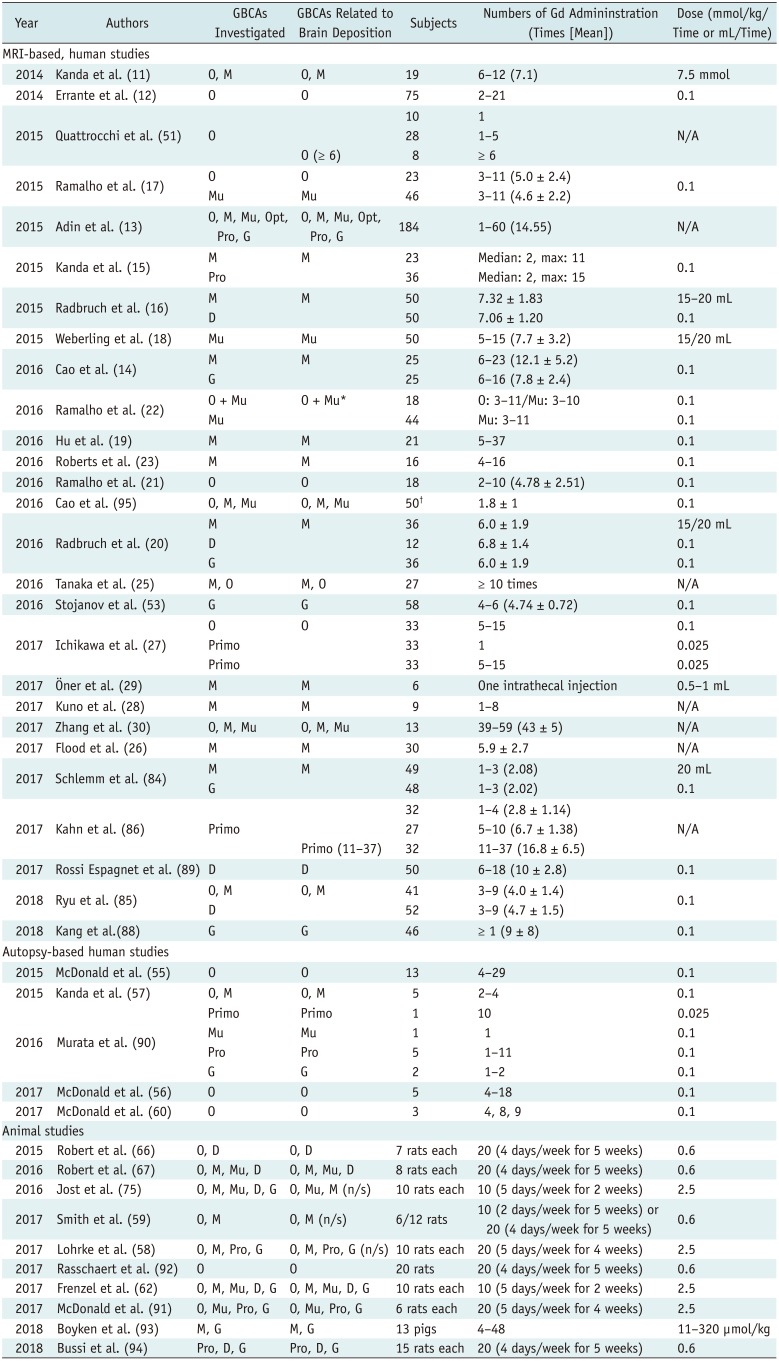




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download