Abstract
Objective
To compare digital breast tomosynthesis (DBT) and conventional full-field digital mammography (FFDM) in the detectability of breast cancers in patients with dense breast tissue, and to determine the influencing factors in the detection of breast cancers using the two techniques.
Materials and Methods
Three blinded radiologists independently graded cancer detectability of 300 breast cancers (288 women with dense breasts) on DBT and conventional FFDM images, retrospectively. Hormone status, histologic grade, T stage, and breast cancer subtype were recorded to identify factors affecting cancer detectability. The Wilcoxon signed-rank test was used to compare cancer detectability by DBT and conventional FFDM. Fisher's exact tests were used to determine differences in cancer characteristics between detectability groups. Kruskal-Wallis tests were used to determine whether the detectability score differed according to cancer characteristics.
Results
Forty breast cancers (13.3%) were detectable only with DBT; 191 (63.7%) breast cancers were detected with both FFDM and DBT, and 69 (23%) were not detected with either. Cancer detectability scores were significantly higher for DBT than for conventional FFDM (median score, 6; range, 0–6; p < 0.001). The DBT-only cancer group had more invasive lobular-type breast cancers (22.5%) than the other two groups (i.e., cancer detected on both types of image [both-detected group], 5.2%; cancer not detected on either type of image [both-non-detected group], 7.3%), and less detectability of ductal carcinoma in situ (5% vs. 16.8% [both-detected group] vs. 27.5% [both-non-detected group]). Low-grade cancers were more often detected in the DBT-only group than in the both-detected group (22.5% vs. 10%, p = 0.026). Human epidermal growth factor receptor-2 (HER-2)-negative cancers were more often detected in the DBT-only group than in the both-detected group (92.3% vs. 70.5%, p = 0.004). Cancers surrounded by mostly glandular tissue were detected less often in the DBT only group than in the both-non-detected group (10% vs. 31.9%, p = 0.016). DBT cancer detectability scores were significantly associated with cancer type (p = 0.012), histologic grade (p = 0.013), T and N stage (p = 0.001, p = 0.024), proportion of glandular tissue surrounding lesions (p = 0.013), and lesion type (p < 0.001).
Conventional mammography is currently the standard modality for breast cancer screening (1). Mammography has been proven to decrease the mortality of breast cancer by as much as 50% (2); however, overlapping fibroglandular breast tissue represents a fundamental limitation of the procedure. This overlap decreases screening sensitivity, particularly in women with dense breasts. Digital breast tomosynthesis (DBT) resolves the issue of overlapping fibroglandular breast tissue, which can obscure a breast cancer or mimic a pseudo-tumor, potentially increasing the sensitivity for detecting breast cancers and decreasing the false-positive rate (3).
Several studies have evaluated the diagnostic performance of DBT. One study reported that DBT increases the sensitivity and specificity of breast cancer detection (4), while another demonstrated that DBT increases the diagnostic accuracy of lesion detection and margin characterization (5). An additional study reported that DBT was significantly superior to two-dimensional full-field digital mammography (conventional FFDM) in evaluating overall lesion size (6). Furthermore, the addition of DBT increases the sensitivity of conventional FFDM mammography in patients with dense breasts, as well as the specificity of conventional FFDM mammography for dense and fatty breasts (7). A recent study suggested that DBT could aid the detection of smaller and less aggressive subtypes of invasive cancers; however, the study only included invasive cancers in fatty and dense breasts (8).
To the best of our knowledge, no studies have analyzed the association between cancer detectability and pathologic or imaging characteristics of breast cancer, including the detectability of ductal carcinoma in situ (DCIS) on DBT images in dense breasts. Therefore, we aimed to investigate whether DBT is superior to conventional FFDM imaging for detecting breast cancer in patients with dense breast tissue, and to determine which characteristics of breast cancers are associated with cancer detectability on DBT images.
The present study was approved by the Institutional Review Board of our hospital, who waived the requirement for obtaining informed patient consent due to the retrospective design of the study. We selected women who had undergone breast surgery and both DBT and conventional FFDM imaging assessments. From April 2014 to December 2015, data of 397 breast lesions in 377 women with dense breasts (composition C and D according to the Breast Imaging Reporting and Data System criteria) were collected for image analysis. Among these, 97 lesions were excluded (Fig. 1).
Finally, 288 women (age range, 27–76 years; mean age, 48.5 years) with 300 lesions were included in our study population. Among them, 276 patients had one lesion, while 12 patients had two lesions (one lesion in each breast). Pathology reports and immunohistochemistry results were collected for all patients, to determine which breast cancer characteristics are associated with the DBT cancer detectability score. These pathologic factors included the type of cancer (DCIS, invasive ductal carcinoma [IDC], invasive lobular carcinoma [ILC], and others), T stage, histologic grade (grades 1–3), and hormone status (estrogen receptor [ER]/progesterone receptor [PR]/human epidermal growth factor receptor-2 [HER-2]). Hormone status was categorized as follows: ER +/−, PR +/−, and HER-2 +/−.
The pathologic characteristics of the study population are presented in Table 1. Most lesions involved IDC (204/300, 68%), followed by DCIS (53/300, 17.7%). T stages were mostly carcinoma in situ (Tis) to T2 (294/300, 98%), indicating that most cancers were < 5 cm in size.
Ten patients underwent conventional FFDM and DBT at separate times, while the remaining patients underwent DBT and conventional FFDM consecutively, with a single breast compression (combo mode). All DBT images and combomode images (bilateral craniocaudal [CC] and mediolateral oblique [MLO] projections) were obtained using a single DBT system (Selenia Dimensions System; Hologic, Bedford, MA, USA). Some conventional FFDM images were acquired using another digital mammography unit (Senographe 2000D FFDM; GE Medical Systems, Buc, France). All images were obtained from each breast with both CC and MLO projections. DBT acquisitions were performed at a combined radiation dose comparable to that utilized in the FFDM examination, with an average mid-breast dose of approximately 1.5 mGy per view.
Magnetic resonance (MR) images were used to assess the location and size of lesions. All patients underwent magnetic resonance imaging (MRI) for detection of multifocal, multicentric, or contralateral breast cancer. MRI was performed using a 3T MRI system (Achieva or Ingenia; Philips Medical Systems, Best, The Netherlands) with a dedicated breast coil.
A total of 10 sets of separate FFDM and DBT images, 278 pairs of combination images (DBT and conventional FFDM images), and 288 ultrasound and MR images from 288 patients were reviewed.
Five radiologists with 1–15 years of experience in breast imaging performed two separate review sessions. First, three radiologists performed blinded reviews, independently. The reviewers were aware that all patients had received a diagnosis of breast cancer, but had no additional clinicopathologic information. Each image analysis session contained four bilateral routine FFDM or DBT images in a randomized order. To minimize memory effects, at least a 1-month interval was allowed between sessions. The reviewer marked arrows on FFDM or representative DBT slices when a tumor was detected. When multiple lesions were observed, only the largest tumor was assessed.
The remaining two non-blinded radiologists retrospectively reviewed DBT images, conventional FFDM images, ultrasound and MR images. Images were interpreted in consensus based on structures surrounding each lesion, and lesion types. We focused only on biopsy-confirmed breast cancers in the present study. The reference standard of breast cancer was based on all breast imaging, including mammography, ultrasound, and MRI, as well as surgical sample pathologic results. First, reviewers checked whether the blindly-reviewed, marked lesions were visible on conventional FFDM and DBT images, and then examined which visible lesions were matched to biopsy-confirmed breast cancers on MR images. If a lesion that was visible on conventional FFDM and DBT images was not matched to that on MR images, the conventional FFDM and DBT images were re-evaluated to locate the appropriate corresponding lesion.
Once the correct lesions had been identified on conventional FFDM and DBT images, each blinded reviewer result was scored as follows: 0, cancer was not marked in any view or was wrongly marked; 1, cancer was marked in only one view: 2, cancer was correctly marked in both views or cancer was marked in only one view when a malignant lesion was seen in only one view. The detectability score was determined as the sum of the three blinded reviewers' scores. If the detectability score was 5 or higher, the malignancy was considered to have been detected. The unblinded reviewers analyzed the structures surrounding a lesion on MR images, after which the type of surrounding tissue was classified as mostly glandular, mostly fat, or equal amounts of each. The degree of glandular tissue surrounding the lesion was then rated from 1 to 10 based on division of 360 degrees (1, 0–36°; 2, 36–72°; 3, 72–108°; 4, 108–144°; 5, 144–180°; 6, 180–216°; 7, 216–252°; 8, 252–288°; 9, 288–324°; 10, 324–360°).
The Wilcoxon signed-rank test was used to compare the detectability scores of cancers identified on DBT and/or conventional FFDM images. We used Fisher's exact tests to determine the differences in cancer characteristics between detectability groups, and the Kruskal-Wallis test to determine whether the detectability score differed according to the cancer characteristics. Statistical analyses were performed using commercial software (Stata 14; Stata Corp, College Station, TX, USA, and MedCalc; MedCalc Software, Mariakerke, Belgium). Differences of p < 0.05 were considered statistically significant.
The image features of breast cancers and detectability are presented in Table 2. Our analysis of mammographic parenchymal density revealed that 199 lesions were heterogeneously dense (199/300, 66.3%), while 101 lesions were extremely dense (101/300, 33.7%).
Detectability scores for DBT images were significantly higher than those for conventional FFDM images (median score, 6; range 0–6; p < 0.001). In total, 191 (63.7%) of 300 breast cancers were detected both on FFDM and DBT (Fig. 2). A total of 40 (13.3%) breast cancers were detected on DBT but were invisible on conventional FFDM images (Fig. 3). The remaining 69 cancers (23%) were not detected on either type of technique (Tables 2, 3, Fig. 4). The DBT-only cancer group had more invasive lobular-type breast cancers (22.5%) than the other two groups (i.e., cancer detected on both types of image [both-detected group], 5.2%; cancer not detected on either type of image [both-non-detected group], 7.3%), and less DCIS (5% vs. 16.8% [both-detected group] vs. 27.5% [both-non-detected group]) (Fig. 3). Low-grade cancers were more often detected in the DBT-only group than in the both-detected group (22.5% vs. 10%, p = 0.026) (Fig. 3). HER-2-negative cancers were more often detected in the DBT-only group than in the both-detected group (92.3% vs. 70.5%, p = 0.004) (Fig. 3). Cancers surrounded by mostly glandular tissue were detected less often in the DBT only group than in the both-non-detected group (10% vs. 31.9%, p = 0.016) (Fig. 4). Mass lesions and architectural distortion were more frequently detected in the DBT-only group than in the other two groups; detectabilities for mass lesions (DBT-only, 90%; both detected, 69.6%; both non-detected, 47.8%; p = 0.001) and those for architectural distortion (DBT-only, 7.5%; both detected, 0%; both non-detected, 1.5%; p = 0.001).
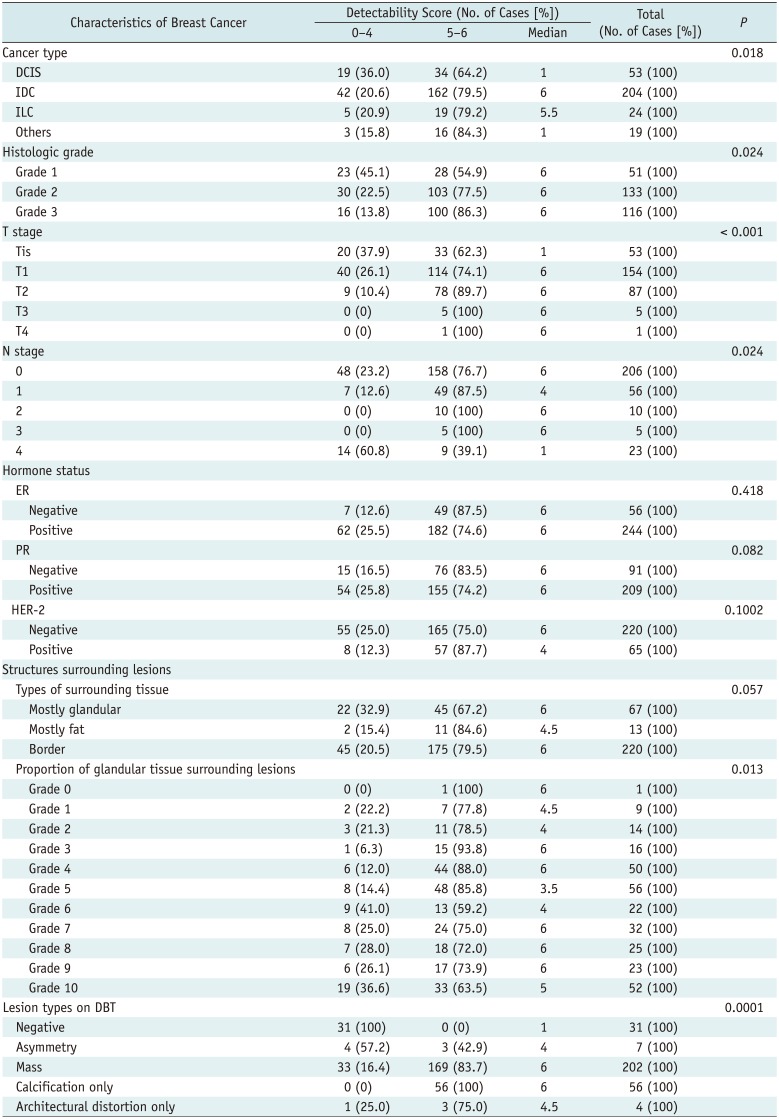
The detectability scores of DBT were significantly associated with cancer type, histologic grade, T and N stage, proportion of glandular tissue surrounding the lesions, and lesion type on DBT images (Table 4). Cancers with higher histologic grades were significantly more visible on DBT images than those with lower histologic grades (p = 0.013). A total of 96 of 116 (82.8%) grade 3 cancers were assigned a detectability score of 6, while 100 of 133 (75.2%) grade 2 cancers and 26 of 51 (51%) grade 1 cancers were assigned a detectability score of 6. Cancers with a high T stage exhibited significantly higher detectability scores, as expected (p < 0.001). Cancers appearing as masses or calcification on DBT images were significantly more visible than those appearing as asymmetry or architectural distortion (p < 0.001).
In the present study, we analyzed differences in cancer detectability between DBT and conventional FFDM images patients with in dense breast tissue. Forty breast cancers (13.3%) were more detectable on DBT and the detectability score was higher on DBT than on conventional FFDM. Our result of 13.3% increase in cancer detection on DBT is comparable to those of single- or multi-center studies, which reported a 10–51% increase in cancer detection (9101112). In our study, 271 of 300 lesions (90.3%) showed a higher detectability score on DBT than on FFDM, which was similar to the results of several previous studies that reported the superiority of DBT over conventional FFDM images (131415). Andersson et al. (13) compared breast cancer detectability between one-view breast tomosynthesis and one- or two-view conventional FFDM, and observed that breast cancer visibility was ranked higher on DBT than on conventional FFDM in 55%. Nam et al. (15) analyzed differences in breast cancer visibility between DBT and conventional FFDM in a group of breast cancers detected via ultrasonography, and reported that these cancers were significantly more constantly visible on DBT (53.7%) than on FFDM (26.8%). Furthermore, 83% of circumscribed masses were reportedly better visualized on DBT images than on conventional FFDM images, particularly in highly dense breasts (14).
Previous studies have also analyzed differences in the characteristics of breast cancers detected on DBT and conventional FFDM images (8121617181920). In our study, 40 breast cancers were detected with DBT only; most of these were invasive (95%, 38/40). This result is similar to those of several previous reports. Bernardi et al. (18) reported that 96% (28/29) of breast cancers detected with DBT were invasive, while only 75% (46/61) of breast cancers detected with conventional FFDM mammography were invasive (18). Wang et al. (17) reported that invasive cancers accounted for 90% (9/10) of the malignancies identified via DBT, while 58% (32/55) of breast cancers identified via conventional digital mammography were invasive (17). Furthermore, invasive lobular-type breast cancers were detected on DBT in our study (22.5% in the DBT only group vs. 5.2% both-detected group vs 7.3% both-non-detected group). Our results were comparable to those of other studies (181921). Skaane et al. (20) reported that the sensitivity increased by 8% when using DBT, and considered that this increase was due to invasive lobular cancer (20). Friedewald et al. (21) reported that there was an increase in detection rates from 0.27 to 0.55 for ILC when tomosynthesis was added. In a study analyzing discrepancies in breast cancer detection on DBT and FFDM, it was found that invasive lobular cancer was more often visible on DBT as spiculated masses, where there was any radiologic findings on FFDM (18). However, other reports showed no difference in detectability according to the type of cancer (812). The populations of invasive lobular cancer included in those studies varied, and therefore further studies including a large sample size of invasive lobular cancer are warranted.
Previous studies have reported inconsistent results in terms of the association between histological grade and detectability on DBT images (81216). Greenberg et al. (12) reported that there were no significant differences in histological grade and type of breast cancer between DBT and conventional FFDM images. In contrast, low-grade cancers were more detectable on DBT (p = 0.026) in our study. This result was comparable to those of some previous reports that showed a significant association between detectability on DBT images and histological grade 1 as 41.7% vs. 12.1% (p < 0.001) in Kim et al. (8) and 70% vs. 27% (p < 0.02) in Wang et al. (17).
Our finding that HER-2 status is significantly associated with DBT detectability (p = 0.004) was comparable to that of a recent study on the biological profiles of invasive cancers detected only on DBT by Kim et al. (8) and they reported that the HER-2 status was significantly associated with DBT-only detection. They also reported luminal A-like subtype was significantly associated with DBT-only detection (8). However, in our study, ER and PR status were slightly higher in the DBT-only group than in the both-detected group (87.5% vs. 77%, 80% vs. 64.4%, respectively), but these factors were not significantly associated with DBT detectability. This might be due to the difference in the study population, as DCIS was included in our study, whereas only invasive cancer was included in the study of Kim et al. (8).
In the present study, we sought to determine whether cancer characteristics are associated with cancer detectability on DBT images. Our findings indicated that cancer type, histologic grade, T and N stage, HER-2 status, and proportion of glandular tissue surrounding lesions were significantly associated with the detectability score. As expected, invasive cancer, higher-grade, HER-2-negative breast cancers, and those with a higher T and N stages were more visible on DBT images. As on FFDM, the obscuring effect of fibroglandular tissue was one of reason for cancers missed on DBT. In the absence of association with architectural distortion, calcifications in the mass, or asymmetry, the proportion of surrounding glandular tissue was a key factor affecting the cancer detectability.
The present study had several limitations of note. First, this was a retrospective, single-institution study. Second, the detectability scores were subjective, although we aimed to provide the most objective measurements possible. Third, we compared the detectability of breast cancer between DCIS and invasive cancers, although the proportion of DCIS cases was relatively small (13.1 vs. 86.9). Nonetheless, our study included a relatively large number of breast cancer cases in which both DBT and conventional FFDM images were obtained in the same individuals.
Despite these limitations, our findings indicated that DBT images were superior to conventional FFDM images with regard to breast cancer detectability in dense breasts, suggesting that DBT may be more effective in screening for breast cancer in patients with dense breasts. Furthermore, invasive lesions, those with a lower histologic grade of HER-2-negative, those being presented as masses, or those with architectural distortion were more detectable on DBT images. Although DBT may improve the detection of breast cancer, it is still limited by the presence of surrounding glandular tissue in dense breasts.
Notes
References
1. Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005; 353:1773–1783. PMID: 16169887.

2. Kopans DB. The most recent breast cancer screening controversy about whether mammographic screening benefits women at any age: nonsense and nonscience. AJR Am J Roentgenol. 2003; 180:21–26. PMID: 12490471.

3. Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol. 2011; 18:1298–1310. PMID: 21893296.
4. Lei J, Yang P, Zhang L, Wang Y, Yang K. Diagnostic accuracy of digital breast tomosynthesis versus digital mammography for benign and malignant lesions in breasts: a meta-analysis. Eur Radiol. 2014; 24:595–602. PMID: 24121712.

5. Yang TL, Liang HL, Chou CP, Huang JS, Pan HB. The adjunctive digital breast tomosynthesis in diagnosis of breast cancer. Biomed Res Int. 2013; 6. 17. [Epub]. DOI: 10.1155/2013/597253.

6. Mun HS, Kim HH, Shin HJ, Cha JH, Ruppel PL, Oh HY, et al. Assessment of extent of breast cancer: comparison between digital breast tomosynthesis and full-field digital mammography. Clin Radiol. 2013; 68:1254–1259. PMID: 23969151.

7. Gilbert FJ, Tucker L, Gillan MG, Willsher P, Cooke J, Duncan KA, et al. Accuracy of digital breast tomosynthesis for depicting breast cancer subgroups in a UK retrospective reading study (TOMMY Trial). Radiology. 2015; 277:697–706. PMID: 26176654.

8. Kim JY, Kang HJ, Shin JK, Lee NK, Song YS, Nam KJ, et al. Biologic profiles of invasive breast cancers detected only with digital breast tomosynthesis. AJR Am J Roentgenol. 2017; 209:1411–1418. PMID: 28834445.

9. Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013; 267:47–56. PMID: 23297332.

10. Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013; 14:583–589. PMID: 23623721.

11. Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R Jr. Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol. 2013; 200:1401–1408. PMID: 23701081.

12. Greenberg JS, Javitt MC, Katzen J, Michael S, Holland AE. Clinical performance metrics of 3D digital breast tomosynthesis compared with 2D digital mammography for breast cancer screening in community practice. AJR Am J Roentgenol. 2014; 203:687–693. PMID: 24918774.

13. Andersson I, Ikeda DM, Zackrisson S, Ruschin M, Svahn T, Timberg P, et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol. 2008; 18:2817–2825. PMID: 18641998.

14. Nakashima K, Uematsu T, Itoh T, Takahashi K, Nishimura S, Hayashi T, et al. Comparison of visibility of circumscribed masses on Digital Breast Tomosynthesis (DBT) and 2D mammography: are circumscribed masses better visualized and assured of being benign on DBT? Eur Radiol. 2017; 27:570–577. PMID: 27236817.

15. Nam KJ, Han BK, Ko ES, Choi JS, Ko EY, Jeong DW, et al. Comparison of full-field digital mammography and digital breast tomosynthesis in ultrasonography-detected breast cancers. Breast. 2015; 24:649–655. PMID: 26292782.

16. Svahn TM, Chakraborty DP, Ikeda D, Zackrisson S, Do Y, Mattsson S, et al. Breast tomosynthesis and digital mammography: a comparison of diagnostic accuracy. Br J Radiol. 2012; 85:e1074–e1082. PMID: 22674710.

17. Wang WS, Hardesty L, Borgstede J, Takahashi J, Sams S. Breast cancers found with digital breast tomosynthesis: a comparison of pathology and histologic grade. Breast J. 2016; 22:651–656. PMID: 27870337.

18. Bernardi D, Macaskill P, Pellegrini M, Valentini M, Fantò C, Ostillio L, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016; 17:1105–1113. PMID: 27345635.

19. Lång K, Andersson I, Zackrisson S. Breast cancer detection in digital breast tomosynthesis and digital mammography-a side-by-side review of discrepant cases. Br J Radiol. 2014; 87:20140080. PMID: 24896197.

20. Skaane P, Gullien R, Bjørndal H, Eben EB, Ekseth U, Haakenaasen U, et al. Digital breast tomosynthesis (DBT): initial experience in a clinical setting. Acta Radiol. 2012; 53:524–529. PMID: 22593120.

21. Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014; 311:2499–2507. PMID: 25058084.

Fig. 1
Flow chart of patient enrollment.
DBT = digital breast tomosynthesis, FFDM = full-field digital mammography, MRI = magnetic resonance imaging
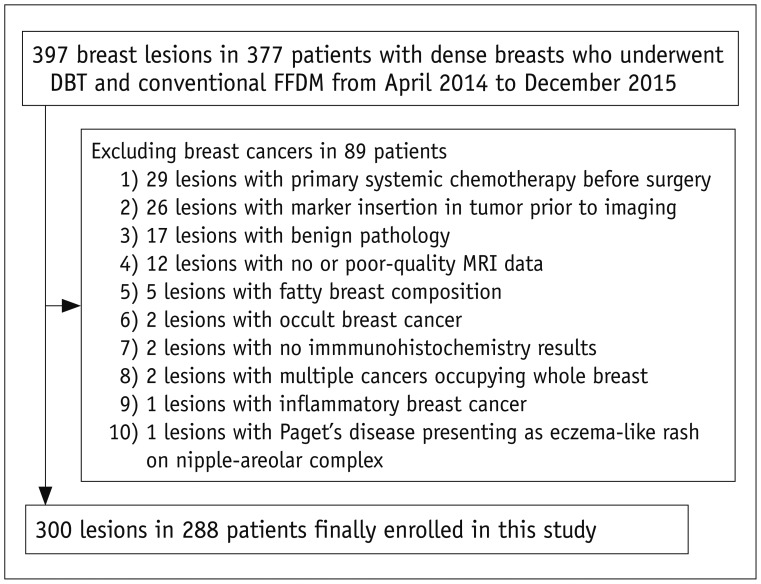
Fig. 2
56-year-old woman with ER/PR-negative, Her-2-positive, high-grade invasive ductal cancer in right breast.
Conventional FFDM (A) and tomography (B) images of right breast in MLO view showing indistinct oval high-density mass with detectability score of 6 (arrows). Irregular rim-enhanced mass (arrow) was located in fat tissue and was 0 degree surrounded on MRI (C). ER = estrogen receptor, HER-2 = human epidermal growth factor receptor-2, MLO = mediolateral oblique, PR = progesterone receptor
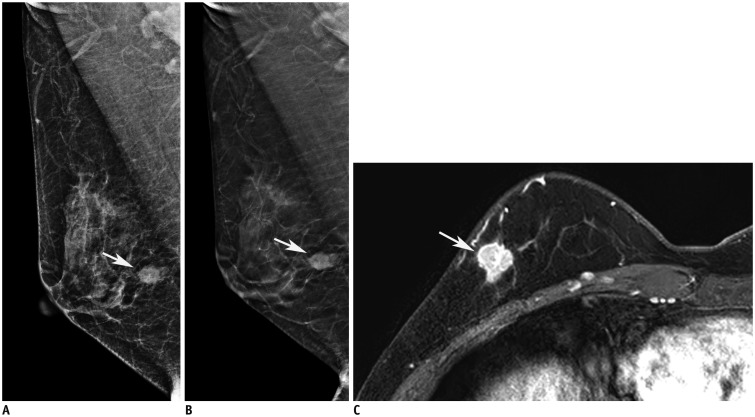
Fig. 3
54-year-old woman with ER-positive, PR/Her-2-negative, low-grade invasive lobular cancer in left breast.
Conventional FFDM of left breast in MLO view (A) showing asymmetry, with detectability score of 2 (arrows). Tomography of left breast in craniocaudal (B) and MLO (C) views showing architectural distortion, with detectability score of 6 (arrows). Regional heterogeneous non-mass enhancement (arrow) was located in fibroglandular tissue and was 7 (240 degrees) surrounded on MRI (D).
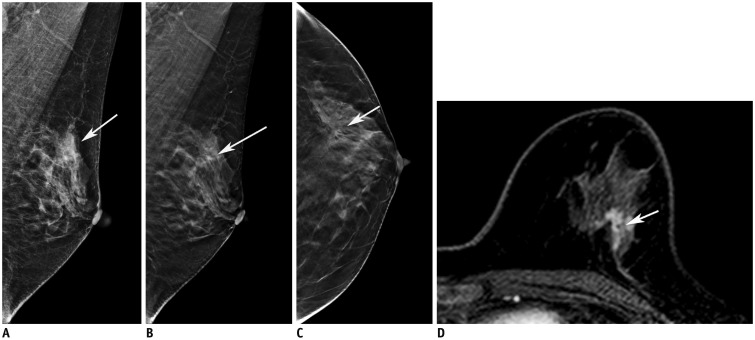
Fig. 4
51-year-old woman with triple negative, high-grade invasive ductal cancer in left breast.
No abnormal findings (detectability score of 0) were noted on either conventional FFDM (A) or DBT (B) images. Mass (arrows) was located in fibroglandular tissue and was 10 (360 degree) surrounded on ultrasonography (C) and MRI (D).
Table 1
Pathologic Characteristics of Breast Cancers
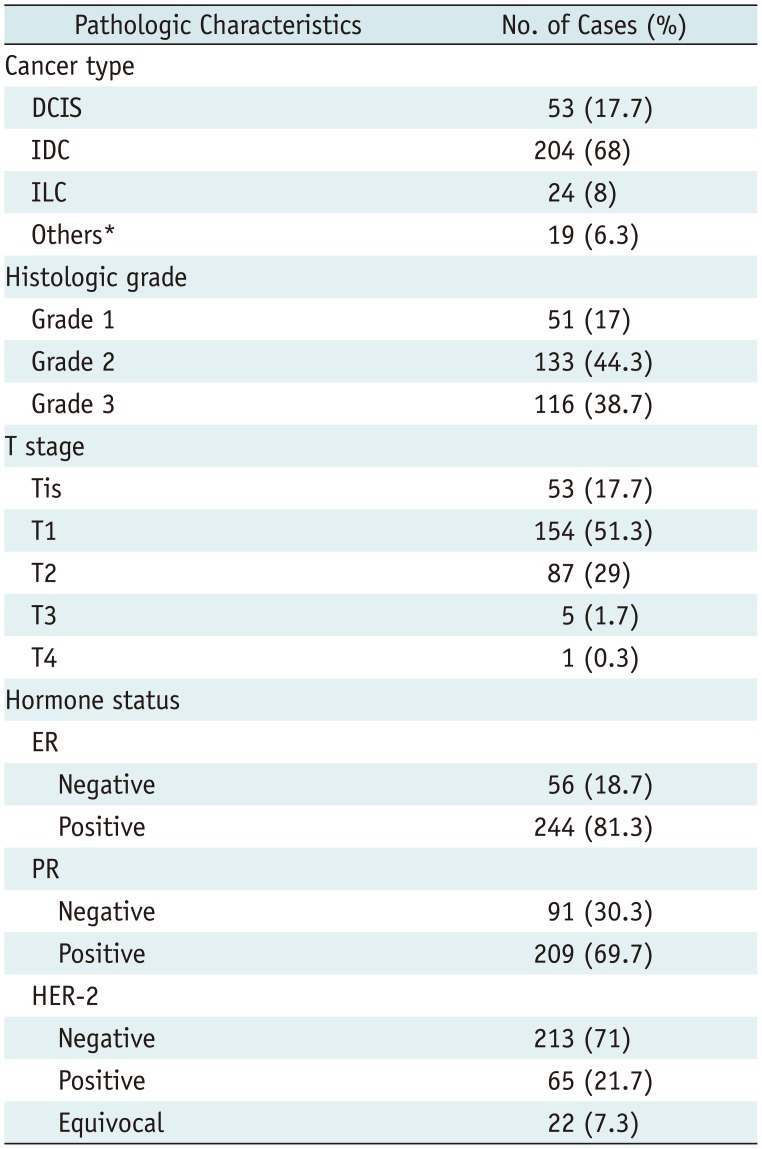
*Others included minor types of breast cancer, such as micropapillary carcinoma or mixed cancer involving more than two types of cancers. DCIS = ductal carcinoma in situ, ER = estrogen receptor, HER-2 = human epidermal growth factor receptor-2, IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma, PR = progesterone receptor, Tis = carcinoma in situ
Table 2
Imaging Features of Breast Cancers and Detectability
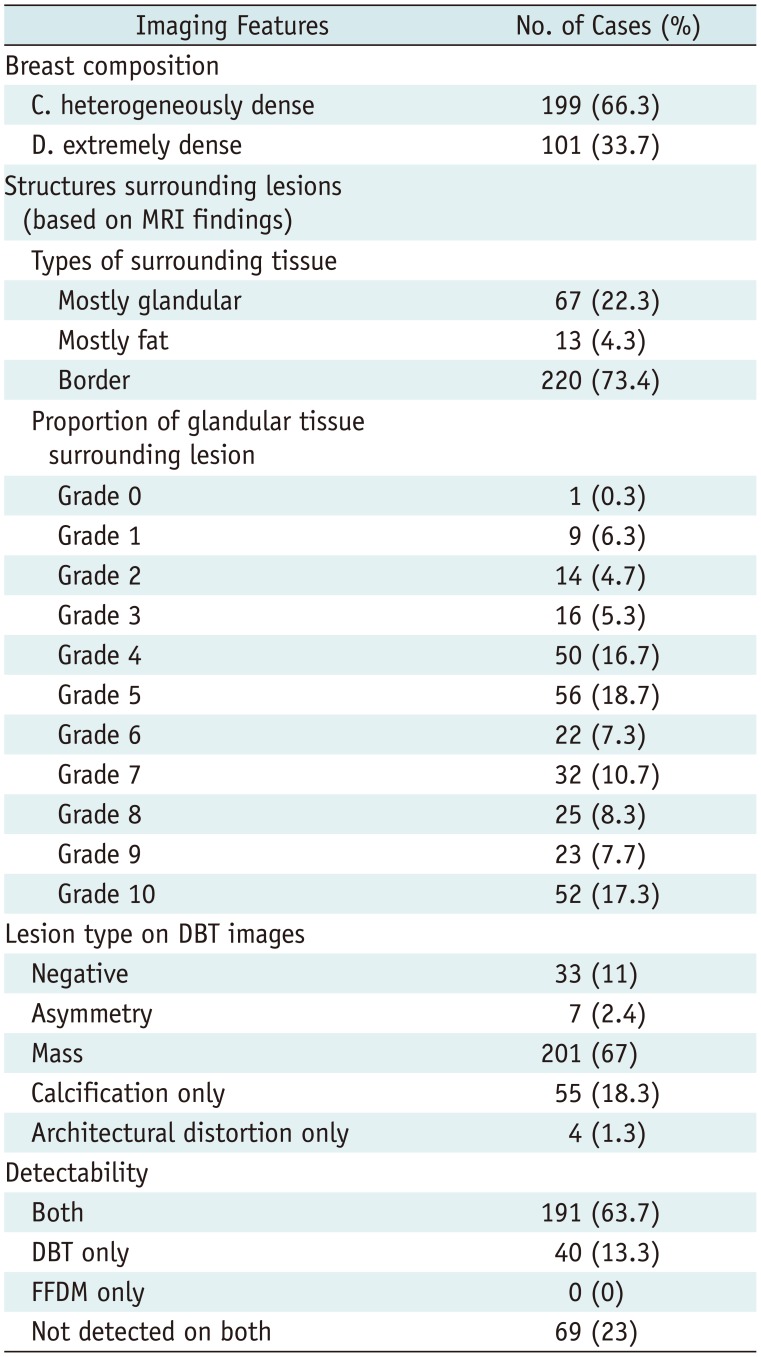
Table 3
Cancer Characteristics according to Detected Modality
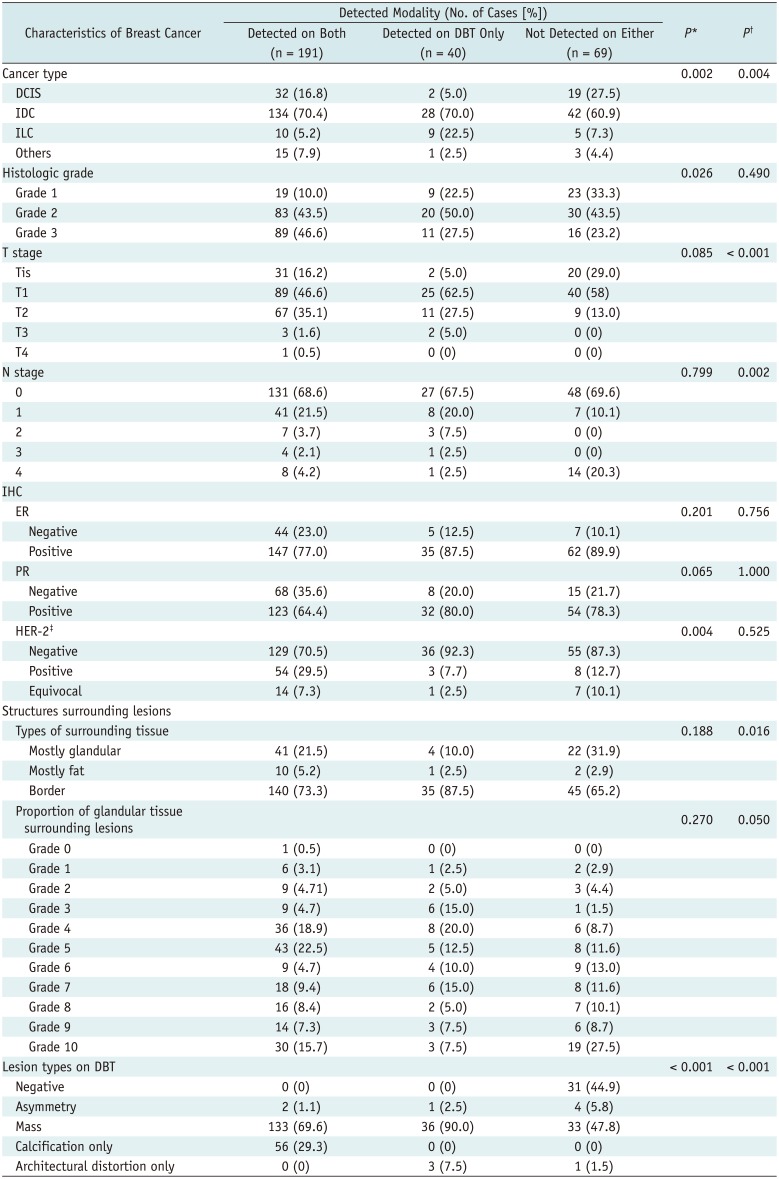
Table 4
Association between Cancer Characteristics and Detectability Score on DBT Images




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download