Abstract
Core needle biopsy (CNB) has been suggested as a complementary diagnostic method to fine-needle aspiration in patients with thyroid nodules. Many recent CNB studies have suggested a more advanced role for CNB, but there are still no guidelines on its use. Therefore, the Task Force Committee of the Korean Society of Thyroid Radiology has developed the present consensus statement and recommendations for the role of CNB in the diagnosis of thyroid nodules. These recommendations are based on evidence from the current literature and expert consensus.
Fine-needle aspiration (FNA), large-needle aspiration biopsy, and large-needle biopsy have all been used for the diagnosis of thyroid nodules (12). In the 1980s, FNA became the standard diagnostic tool for the thyroid, replacing large-needle biopsy, because of its high diagnostic accuracy and low complication rate (3). Therefore, large-needle biopsy, performed without ultrasound (US) guidance with a large-bore needle, is not currently recommended for thyroid nodules because of local pain and risk of cervical bleeding (45). Although FNA shows a high diagnostic specificity and safety, it has several limitations: 1) an average reported diagnostic sensitivity of about 83% with a false-negative rate of 2–18% (567); 2) a non-diagnostic rate in initial FNA of about 10% and an even higher rate of up to 50% in repeat FNA (89); 3) a rate of atypia (follicular lesion) of undetermined significance of about 10–20% with high rates of inconclusive results in repeat FNA, specifically, a 1–7% non-diagnostic rate and 3.8–31.0% of atypia (follicular lesion) of undetermined significance (101112); and 4) low diagnostic accuracy for follicular lesions (1314). These limitations of FNA lead to repeat FNA or unnecessary surgery (15). Therefore, additional diagnostic tools are necessary to overcome the limitations of FNA for thyroid nodules.
With advances in core biopsy devices, spring-activated single- or double-action needles have been applied to thyroid nodule diagnosis. In addition, widespread use of high-resolution US enables accurate diagnosis and minimization of complications (16). Therefore, core needle biopsy (CNB) has been reported to be an effective and safe biopsy method for thyroid nodules (1718192021). CNB has the potential to overcome the limitations of FNA by obtaining a large amount of tissue from the nodule, providing more information on architectural histologic structures, including the nodule capsule, and permitting feasible immunochemical staining (1822232425). Such a role for CNB has been suggested in many recent articles (181922). However, its indications, basic technique, and safety remain unclear.
In 2013, the Korean Society of Thyroid Radiology (KSThR), an organization of thyroid radiologists in Korea primarily involved in the diagnosis and non-surgical treatment of thyroid nodules (2627282930), proposed the first set of CNB recommendations for thyroid nodules (31). These recommendations were formulated by an organized task force committee that included several specialists in thyroid CNB. The recommendation content included patient selection, indications, efficacy, and safety and the guidelines have been widely used in Korea. Because new information has become available since 2013 from clinical studies of CNB in patients with thyroid nodules, the task force committee members suggested the need to revise these first recommendations. Accordingly, the KSThR organized a committee for this purpose, and this committee has been preparing recommendations for CNB of thyroid nodules since July 4, 2014. The revised recommendations include sections dealing with indications, devices, terminology, preprocedural evaluations, CNB procedures, efficacy, pathologic criteria, safety, and conclusions. The scope, information identification methodology, and availability are described in Tables 1, 2, 3. A PubMed, Medline search was performed with the keywords "core needle" and "thyroid" up to May 2016. We used the Delphi survey technique to reach a consensus and enhance effective decision making. The recommendations are summarized in Table 4 with the opinions of the Delphi survey members. Because there is little high-level evidence, some of these recommendations are based on expert opinion. This limitation needs to be overcome in the future (e.g., within 3–5 years) after further research.
Thus, the goal of these recommendations was to provide the best scientific evidence available and a consensus expert opinion regarding the use of CNB of the thyroid in clinical practice.
Although the indications for CNB remain not to be clearly defined, most guidelines suggest CNB as a complementary tool to FNA rather than the first-line diagnostic tool. Before 2014, there was little information from current guidelines on CNB. The National Cancer Institute (NCI), American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi (AACE/ACE/AME), and KSThR have proposed CNB for thyroid nodules with previous non-diagnostic FNA results (53132). The NCI and KSThR have suggested a role for US-guided CNB using spring-activated core biopsy needles in cases deemed "unsatisfactory/non-diagnostic" in FNA; however, CNB was not considered a competitor to FNA, but rather a complementary tool (32). The KSThR has also suggested the possible use of CNB for thyroid nodules with indeterminate FNA results. The AACE/ACE/AME, British Thyroid Association, and KSThR suggested the use of CNB for some malignant thyroid tumors (i.e., lymphoma, anaplastic cancer, medullary cancer, and metastasis) (53133). The American Thyroid Association (ATA) did not recommend the use of CNB for thyroid tumors (34).
Based on the current evidence, CNB has been suggested as an alternative to repeat FNA for thyroid nodules with previous non-diagnostic results (182135). CNB could reduce the rate of non-diagnostic results or diagnostic surgery in calcified thyroid nodules (3637). In addition, several studies showed that CNB could be a useful next-management tool for thyroid nodules with previously atypia (follicular lesion) of undetermined significance in FNA (18193839). When thyroid lymphoma or anaplastic cancer is suggested, CNB is preferable to FNA in many studies (16404142). A recent study suggested a role for CNB in medullary thyroid carcinoma (43).
[Recommendation 1]
Core needle biopsy could be an alternative to FNA in the evaluation of thyroid nodules in selected cases.
Fine-needle aspiration uses finer needles (21–27 gauges) than the large-bore needles of large-needle aspiration (14 gauge). Recently introduced CNB devices have a smaller bore (usually 18–21 gauge) and spring-activated needles (184445). Large-needle biopsy, performed without US guidance with a large-bore needle, is not recommended for thyroid nodules.
The proper CNB needle conditions for thyroid nodules are the following. First, the entire length of needle should not exceed 10 cm because the thyroid gland is a superficial organ. Second, needle thickness, especially inner diameter, determines the thickness of the specimen. However, there is still no evidence supporting the choice of needle gauge. Some studies report the use of 16–22-gauge needles (1821454647). Although the use of 18–21-gauge needles is universal for thyroid nodules, 18-gauge needles have been mainly used in Korea (1819484950). The thinner the needle, the less damage to normal tissue but the lower the amount of tissue obtained. CNB needles for thyroid nodules typically have the following characteristics: diameter, 18–21 gauge; needle length, 6–10 cm; excursion length, 1.1–2.0 cm. However, there is no evidence regarding the relationship between needle thickness, complication rate and diagnostic accuracy. Finally, the length of the stylet, namely, the penetration length, can be selected according to the size of nodule and is usually 1–2 cm.
Core needle biopsy needles are composed of two needles, the stylet and the cutting cannula (Fig. 1) (1844). The stylet or inner needle has an approximately 2-mm-long sharply sloped tip to penetrate tissue and a specimen notch for holding the sampled tissue. The cutting cannula or the outer blade is the outer component of the CNB needle and plays a role in cutting the tissue and placing it on the specimen notch. CNB needles are divided into two types according to the mechanism of action: automated and semi-automated. The automated needle is called a double-action needle because both inner and outer needles are spring-activated. This type of needle fires the stylet via a spring action that can more easily penetrate hard tissue. However, it may be more prone to adjacent tissue damage. The semi-automated needle is called a single-action needle because the spring activates just once: the stylet is manually introduced, followed by the spring-activated needle. The semi-automated needle enables a relatively safe procedure, despite the presence of a risky aspect because operators manually push the stylet into the tissue. There are various kinds of spring power. Although a device with a strong spring can better penetrate hard tissues such as calcifications or fibrosis, it has higher potential for injury of normal tissue or vessels. The amount of tissue obtained depends on the needle thickness and the length of the specimen notch (51).
The guiding needle or coaxial needle is a separate needle that provides assistance and easy intraneedle passage of the core needle to the target. It provides a clear path to work through when performing multiple biopsies in the same area and can improve accuracy and efficiency. However, the needle tract is greater because the size is larger than that of the corresponding biopsy needles.
[Recommendation 2]
Modern CNB devices, particularly 18–21-gauge, spring-activated, core needles, are recommended for the procedure.
Fasting is not recommended for CNB in standard conditions (31). Informed consent should be obtained from all patients after discussion with them of the purpose, procedure, possible complications, and need for CNB. The use of drugs, especially those that affect bleeding tendency, such as warfarin, heparin, aspirin, or clopidogrel bisulfate, should be checked. Interruption of such agents can reduce the risk of biopsy-related complications. Bleeding tendency is checked by communication with patients and a screening blood test for coagulation is usually unnecessary. Aspirin and clopidogrel bisulfate should be withdrawn for 7–10 days, warfarin for 3–5 days, and heparin for 4–6 hours before the procedure. After the procedure, aspirin and clopidogrel bisulfate can be started from the next day, warfarin that night, and heparin 2 hours later (52). However, anticoagulant withdrawal should be carefully discussed with the prescribing physician. Warfarin can be transiently changed to shorter-acting heparin (52).
[Recommendation 3]
Patients with bleeding tendency, such as those taking anticoagulation medications or with disorders affecting the coagulation cascade, should be thoroughly evaluated and any problems corrected before CNB.
Core needle biopsy should be performed by experienced operators under US guidance. Operators should determine the appropriate type of CNB needle and access route via preprocedural US evaluation, which is also important for improving safety and diagnostic accuracy (31). Although no standard technique for thyroid CNB has been established, the KSThR recommend the following techniques for effective and safe procedures.
Experience is one of the most important factors for CNB safety. Less-experienced operators may have difficulties in finding the needle tip under US, which can increase the possibility of complications. CNB procedures by less-experienced operators should be supervised by experienced operators.
During the US-guided procedure, there are two options for the guidance of CNB needles: a free-hand technique or a US probe-guiding device. Our society recommends a free-hand technique because it allows greater freedom, permitting the operators both to select the puncture point and to adjust the route during the procedure (51). Before the procedure, the nodule size, position, characteristics, and vascularity should be evaluated by gray-scale and color Doppler US. Vascular injury can be minimized by the use of color Doppler US guidance during the procedure. The operator should decide the CNB approach route using the information from the preprocedural US evaluation. Approximately four approach routes are available: transisthmic, lateral, longitudinal, and oblique. The most suitable approach has been suggested to be the transisthmic approach. It is recommended to choose a needle with a similar specimen notch length to nodule diameter to minimize the sampling of normal tissue (1822).
Patients lie down in a supine position with their neck fully extended. Local anesthesia with 1% lidocaine applied using a finer needle through the route is recommended. Figure 2 shows a CNB procedure on US. To pass through the skin and thyroid capsule, a snapping movement of the wrist is favored for effective rapid needle passage and to reduce pain.
Skin puncture can be effectively performed without skin incision by introducing the needle through the entry hole created by the needle or by direct skin puncture with a rapid snapping movement of the wrist with the core needle. The entire length of the CNB needle must be monitored during the procedure and the needle should remain parallel to the axis of the US probe during the procedure, which is mandatory for the optimal US guidance of CNB. A vertical approach of the needle should be avoided because the entire length of the needle cannot be visualized by US during the procedure. Complete vessel mapping along the approach route (from the skin to the nodule) via color Doppler US is crucial to avoid vessel injury and ensure a safe CNB procedure.
When using a double-action CNB needle, ensure that the entire length of the needle (including the needle tip) is visualized as a single plane before stylet firing, and the anticipated moving distance of the stylet should be exactly estimated before firing. The needle tip must be in a safe place, in most cases within the thyroid capsule, and there must be no large vessels within the imagined firing route. Once all of these aspects are checked and the operator is certain of the safety of the imagined route, the stylet can be fired followed by cutting cannula. When a successive method is used, the location of the specimen notch can be adjusted after stylet firing to select the most appropriate sampling site. For pathological interpretation, the three following components should be obtained: nodule tissue, nodule-parenchyma border (sometimes there is a visible capsule), and normal thyroid parenchyma (4651). While adjusting the specimen notch position, blood can rapidly fill the specimen notch if there is a hypervascular nodule. In this case, the CNB might not harvest any adequate tissue, only blood. Simultaneous or rapid firing of a cutting cannula can prevent this kind of blood filling. When a single-action needle is used, the stylet is manually advanced to the margin of the nodule or within the nodule. After adjustment of the location of the stylet and specimen notch, the cutting cannula is fired in the same fashion as for the double-action needle.
Nodules found in a dangerous location should be carefully evaluated. When the nodule is located near the thyroid capsule, placement of the stylet tip outside the capsule is very cautiously allowed because vascular injury and massive bleeding may occur when the specimen notch is located outside the thyroid capsule. In this situation, a single-action needle is safer because fine adjustment of the specimen notch position is possible. After confirmation that the specimen notch is within the thyroid capsule, cutting cannula firing can follow. CNB may also be technically difficult when there is a small nodule located in the deep posterior area of the thyroid gland. In this situation, first insert a stylet into the nodule by manually advancing the stylet and then elevate the nodule with the inserted stylet. The direction of firing would be changed after positioning the stylet to a safer lateral or oblique lateral direction and, then, the cutting cannula can be safely fired (Fig. 3).
Sometimes, thyroid nodules containing severe fibrosis and/or heavy calcification may be too hard to be penetrated by a CNB needle. To successfully obtain samples from such hard nodules, operators can try to stab the nodule with a needle tip to identify a weak point, use a double-action needle with spring power, and/or adjust the specimen notch direction. Sometimes, the nodule is so hard that the needle may be deflected and damage adjacent structures. Thus, before sampling hard nodules, careful evaluation of the surrounding structures is vital (363753).
Biopsy techniques using a guiding needle (coaxial needle) are useful for repeat CNB. In this technique, the biopsy needle is inserted through the lumen of the guiding needle, which has been placed from the skin to the near surface of the nodule. After local anesthesia to the entry point on the skin, the guiding needle is inserted and placed so that the tip is just in front of the near surface of the nodule. The stylet of the guiding needle is removed and the biopsy needle is inserted. The stylet is fired first, then the cutting cannula to obtain the biopsy sample. To obtain any additional samples, the biopsy needle is removed to harvest the tissue sample, leaving the guiding needle in place. Biopsy needle re-insertion and sample collection can be performed through the lumen of the remaining guiding needle. The use of guiding needles has the following advantages: 1) the tip of the guiding needle is sharper, so skin and thyroid capsule penetration is easier; 2) multiple sampling can be done easily via the single insertion of a guiding needle; 3) complications are minimized by reducing the number of repeat thyroid punctures; and 4) finer manipulation of the guiding needle allows the biopsy needle to avoid any critical structures near the nodule.
After biopsy, visual assessment of the tissue sample can help to determine if additional CNB is required (15182237). An important advantage of CNB over FNA is the possibility for visual assessment of the tissue obtained. To the naked eye, normal thyroid parenchyma is seen as blood-red soft tissue. Most cancers, especially when they are solid and fibrous, are seen as hard and whitish tissue. Calcifications are seen as milky whitish tissue.
Therefore, the adequacy of the CNB specimen can be determined via visual assessment. After visual assessment, the harvested tissue should be immediately fixed in formalin. One or two biopsy sampling is sufficient for the adequate histology diagnosis in most thyroid nodules. If the specimen size is too small or inadequate by visual assessment, additional biopsy should be considered. When a nodule has heterogeneous components on US, it is advised to sample tissue from multiple sites of the nodule to represent all areas of the nodule. When there is a complication, such as bleeding, additional sampling can be postponed. Manual compression should be performed immediately after the biopsy for 20 to 30 minutes.
[Recommendation 4]
(A) CNB should be performed by experienced operators under US guidance.
(B) Manual compression of the biopsy site should be performed immediately after the procedure for 20 to 30 minutes.
Although US-guided FNA has been established as an accurate diagnostic method for thyroid nodules, non-diagnostic results are a diagnostic limitation of FNA (554). Non-diagnostic FNA results show a broad range of malignancy rates. Because non-diagnostic aspirates are common causes of false-negative FNA results, the current guidelines recommend repeat FNA under US guidance, although approximately 9.9–47.8% will once again be non-diagnostic (58955). Surgery is recommended for solid nodules with repeated non-diagnostic results for diagnostic purposes (534).
The rates of non-diagnostic results of CNB vary from 1.1% to 7.2% (15181921224756575859). However, three studies reported a non-diagnostic result rate of more than 10% (13%, 23%, and 40.6%, respectively) (43560). Several current guidelines, such as those of the NCI, AACE/ACE/AME, and KSThR, suggest CNB for thyroid nodules with repeated non-diagnostic FNA results (313261). However, the guidelines do not recommend CNB as a first-line diagnostic tool. CNB has been suggested as an effective diagnostic tool for thyroid nodules with previous non-diagnostic results in FNA (1835). Four studies compared the effectiveness of CNB and repeat FNA for thyroid nodules with initially non-diagnostic FNA results (18355657). In these studies, CNB achieved lower non-diagnostic results than repeat FNA. Furthermore, CNB can reduce inconclusive results and unnecessary surgery (1518). One study showed that repeat FNA was the most significant factor for second non-diagnostic biopsy results, although non-diagnostic results of CNB were not related to cystic component, calcifications, or other US features (56). These results suggest that CNB can be used instead of repeat FNA as the subsequent diagnostic approach for thyroid nodules with initially non-diagnostic FNA results. The combination of repeat FNA and CNB has also been suggested, with this combination achieving significantly better results than FNA alone (35).
In recent systematic review and meta-analysis studies, CNB is suggested as an effective diagnostic tool to reduce inconclusive results in thyroid nodules with previously non-diagnostic FNA result (62636465). In these studies, CNB showed lower non-diagnostic or inconclusive results than repeat FNA.
Main causes of non-diagnostic results in CNB are a fibrotic nature of the thyroid nodule or a targeting error. Fibrosis of thyroid nodules is prominent in thyroid nodules with degeneration (37). Targeting errors are usually caused by inexperienced operators, small thyroid nodules, deep nodules, and heavily calcified nodules (1837). Pathological analysis of specimens obtained after a targeting error reveals the presence of normal thyroid tissue only, skeletal muscle, or adipose tissue (60).
[Recommendation 5]
Core needle biopsy can be used as an alternative to FNA for thyroid nodules with non-diagnostic cytology in previous FNA.
One of the limitations of FNA is atypia (follicular lesion) of undetermined significance, which has been reported in about 10–20% of FNA biopsies. The guidelines recommended repeat FNA for these nodules but repeat FNA also shows a non-diagnostic rate of 1–7% and a rate of repeated atypia (follicular lesion) of undetermined significance of 3.8–31.0% (101112). The malignancy risk of the atypia (follicular lesion) of undetermined significance category is 15–25% according to the Bethesda system (54). However, there has been a tendency to overuse the diagnosis of atypia (follicular lesion) of undetermined significance and the reported malignancy risk varies from the proposed rate, from 14% to 38% (6667). To improve the accurate detection of malignancy in these lesions, several solutions have been suggested, such as the use of CNB, BRAF mutations, gene expression classifiers, and a combination of US findings (18216869707172). There are no clear guidelines on the management of atypia (follicular lesion) of undetermined significance. AACE/ACE/AME guidelines do not recommend either in favor of or against the use of CNB in nodules with indeterminate cytology because of the limited evidence and the lack of established reporting systems (5). A recent CNB study showed that subcategory nodules of nuclear atypia had higher risk of malignancy, of becoming surgical candidates, of having malignant US findings, and of having malignant CNB readings than subcategory nodules of architectural atypia (69). Other studies suggested that CNB was helpful for subcategory nodules of nuclear atypia but not (or less helpful) for subcategory nodules of architectural atypia (394873). However, another study of 153 consecutive patients suggested that CNB might be more useful for management decisions than repeat FNA in both subcategory nodules of nuclear atypia and subcategory nodules of architectural atypia and has the potential to be a first-line alternative diagnostic tool in initially diagnosed atypia (follicular lesion) of undetermined significance (39).
The recently revised ATA management guidelines proposed that repeat FNA or molecular testing be used and that, if the results are inconclusive again, surgical excision be performed with consideration of clinical and US features and patient preferences (34). Application of immunohistochemical stains such as galectin-3, cytokeratin-19, Hector Battifora and mesothelioma 1 (HBME-1), and BRAFV600E(VE1) to CNB specimens from thyroid nodules with prior indeterminate FNA reports has been tried (2572). It is unclear whether the addition of immunohistochemical stains would significantly improve diagnostic accuracy for all thyroid nodules. However, immunohistochemical stains seem to be effective for thyroid nodules with indeterminate CNB results (after initial atypia [follicular lesion] of undetermined significance in FNA) (2572).
Several recent studies have shown the usefulness of CNB for thyroid nodules with previous atypia (follicular lesion) of undetermined significance. In a retrospective study comparing three management tools (CNB, repeat FNA, and diagnostic surgery for previous atypia [follicular lesion] of undetermined significance in FNA), the CNB results were better (77.8% benign, 20.3% cancer, and 1.8% non-diagnostic) than those of repeat FNA (35.2% benign, 16.1% cancer, and 48.6% non-diagnostic) and comparable with those of diagnostic surgery (19). In a prospective study of concurrent CNB and FNA, the incidence of non-diagnostic or atypia (follicular lesion) of undetermined significance was lower in CNB (3.1% non-diagnostic and 23.6% atypia [follicular lesion] of undetermined significance) than in repeat FNA (9.3% non-diagnostic and 39.8% atypia [follicular lesion] of undetermined significance) (18).
[Recommendation 6]
Core needle biopsy may be used as an alternative to FNA for thyroid nodules with atypia (follicular lesion) of undetermined significance in previous FNA.
The preoperative diagnosis of follicular neoplasm in the thyroid gland is challenging because some thyroid nodules with Bethesda IV in FNA are non-neoplastic nodules such as nodular hyperplasia and chronic thyroiditis (50). In particular, FNA cannot distinguish follicular carcinoma from follicular adenoma because their differentiation is based on the histologic evaluation of surgical specimens. In nodules with Bethesda category IV, FNA obtains a retrospective neoplasm rate of 60–80% and malignancy rate of 20–40% (124974). CNB has been introduced as a complementary method for thyroid nodules because the large amount of specimen obtained can facilitate more detailed histologic evaluation and ancillary immunohistochemical staining (182324). Moreover, Nasrollah et al. (46) have suggested a new sampling technique that includes the capsule of the nodule and the surrounding extranodular parenchyma, as well as nodular tissue (75). This technique could allow follicular neoplasm and unencapsulated non-neoplastic nodules to be distinguished by identifying the presence of a fibrous capsule on histologic evaluation.
Core needle biopsy is not recommended by current guidelines for the differentiation of follicular adenoma and carcinoma (532). However, the differentiation of follicular neoplasm from nodular hyperplasia is also important for avoiding unnecessary surgery in clinical practice. The management strategy is surgery for follicular neoplasm, but the follow-up strategy more closely resembles that of nodular hyperplasia (5054). There have been few previous studies of the diagnostic value of CNB for follicular neoplasm of the thyroid gland (4950). Yoon et al. (50) reported that CNB was superior to FNA for the diagnosis of follicular neoplasms in terms of the false-positive neoplasm rate (4.7% vs. 30.8%), unnecessary surgery rate (3.7% vs. 26.2%), and malignancy rate (57.9% vs. 28.0%). The researchers believe that CNB can play a role in reducing unnecessary surgery and increasing diagnostic confidence for patients with follicular neoplasm. In contrast, Min et al. (49) determined that CNB was not superior to FNA in the prediction of malignancy (malignancy rate, 46% vs. 48%, p > 0.05). The role of CNB in the diagnosis of follicular neoplasm is still debatable. Therefore, a large prospective study is required to validate the diagnostic efficacy of CNB for follicular neoplasm.
[Recommendation 7]
(A) CNB has advantages to differentiate encapsulated follicular neoplasms from non-neoplastic nodule.
(B) CNB cannot differentiate follicular thyroid carcinoma from follicular adenoma.
Calcified nodules of the thyroid gland are frequently encountered and calcification has been reported to be an important factor related to FNA failure (3776). Restriction of free needle movement during FNA may hinder the collection of adequate and accurate cytological specimens from calcified nodules. CNB has a technical failure rate of 1.1% for the collection of tissue from dense calcified thyroid nodules and a non-diagnostic rate of 0.7–7.7% and could prevent unnecessary diagnostic surgery (363753). CNB obtains fewer inconclusive diagnoses than FNA and could minimize the need for diagnostic surgery in patients with calcified thyroid nodules (3653).
[Recommendation 8]
Core needle biopsy may be used as an alternative to FNA for calcified thyroid nodules.
The utility of CNB has commonly been highlighted for thyroid nodules with previous non-diagnostic results or atypia (follicular lesion) of undetermined significance in FNA. However, only three studies used CNB as the first-line approach for thyroid nodules. These studies enrolled 31, 369, and 632 thyroid nodules, respectively (475977). As a first-line diagnostic tool, CNB reported a high diagnostic accuracy (97.0–97.7%), low non-diagnostic rate (1.1–3.2%), low rate of false-negative results (0–3.9%), and low complication rate. In addition, Trimboli et al. (59) emphasized the high diagnostic accuracy of CNB compared with FNA (96.8% vs. 78.0%, p < 0.05). Zhang et al. (47) showed that CNB had a high rate of conclusive and accurate diagnosis in thyroid nodules with suspicious US findings. This study achieved a high rate of conclusive CNB results regardless of nodule size, vascularity, and US features. Therefore, CNB could reduce repeat biopsy, diagnostic surgery, and unnecessary follow-up (4777). Suh et al. (77) reported a role for CNB in initially detected thyroid nodules in a large cohort (n = 632). Based on subgroup analysis, they stressed that diagnostic performance was not significantly associated with nodule size and that there were no independent risk factors associated with inconclusive results.
Current evidence suggests that CNB may be considered an effective diagnostic tool for initially detected thyroid nodules, especially for thyroid nodules with suspicious US features (4759). Despite these excellent diagnostic performances, more evidence from large well-designed studies are necessary before CNB can be considered a first-line diagnostic tool because previous studies were performed in specific populations, such as in patients with thyroid nodules with suspicious US features, in single centers, and with retrospective designs. In conclusion, CNB may be used as a first-line diagnostic tool for initially detected thyroid nodules with suspicious US features. However, the evidence is still insufficient.
[Recommendation 9]
Core needle biopsy may achieve low rates of non-diagnostic and inconclusive results for initially detected thyroid nodules. However, the utility of CNB as a first-line diagnostic tool for these nodules is uncertain based on current evidence.
Application of CNB to thyroid nodules in children is rare due to the rarity of thyroid nodules in the pediatric population. In addition, most (67.3%) incidentally detected thyroid lesions in children are cysts (78). Only a single institute has reported its experience with CNB (60). This study showed that CNB had relatively high non-diagnostic (13%) and inconclusive (30%) rates. The sensitivity and specificity for malignancy were 88% and 85%, respectively. The results suggested that CNB is comparable to FNA in a similar patient population. CNB has an advantage over FNA in that it can reduce the misinterpretation of parathyroid lesions as follicular neoplasms of the thyroid and decrease the false-positive diagnosis of Hashimoto's thyroiditis that leads to unnecessary thyroidectomies.
Core needle biopsy has been reported to be useful for the specific diagnosis of some malignant thyroid lesions, especially lymphoma, anaplastic carcinoma, and medullary carcinoma (16264379). Both anaplastic thyroid cancer and thyroid lymphoma can be present as rapidly growing thyroid masses in elderly patients (80). Because these tumors have distinct therapeutic strategies and prognoses, they should be accurately differentiated from each other and from other types of thyroid cancers. In a single-center study of 104 patients with anaplastic thyroid cancer and thyroid lymphoma, CNB was suggested to reduce the rate of unnecessary diagnostic surgery by achieving higher diagnostic sensitivity and positive predictive values than FNA. In a large multicenter study of 191 patients with medullary carcinoma, CNB also achieved higher sensitivity and positive predictive values than FNA. Therefore, CNB could be a complementary diagnostic tool to optimize the surgical management of patients with clinically suspected medullary carcinoma (43).
Ultrasound–cytology discordance is a challenge in the management of thyroid nodules. The KSThR previously suggested repeat biopsy for US–cytology discordant nodules (30). Because the risk of malignancy is much higher (20.4–56.6%) for suspicious nodules than those with benign US features (< 3%), a mismatch between US and cytological findings results in a patient being subjected to repeat FNA or even diagnostic surgery (818283). However, benign thyroid nodules, which contain fibrotic and hemorrhagic tissue, can also show suspicious US features in nodules with degenerative changes after FNA/CNB, with intranodular bleeding, or after ethanol and radiofrequency ablation (84858687888990). Degenerative changes can replace the entire thyroid nodule or focal area of nodules (8485). CNB has an excellent diagnostic performance in the management of US–cytology discordant nodules and improves diagnostic confidence via the histologic information obtained from CNB specimens (848590).
Hyalinizing trabecular tumors pose a challenge to diagnosis due to frequent cytological diagnosis of papillary thyroid carcinoma after FNA (919293). Several studies suggested a role for CNB in this rare thyroid tumor (9194). In four cases reported by Choi et al. (91), CNB was a reliable approach to the proper diagnosis of all cases. When CNB does suggest hyalinizing trabecular tumor, an additional immunohistochemical stain such as Ki-67 and cytokeratin 19 can differentiate hyalinizing trabecular tumors from papillary thyroid carcinoma.
[Recommendation 10]
(A) CNB can be used as an alternative to FNA in patients with clinical and radiological features of uncommon malignancies (anaplastic carcinoma, lymphoma, or medullary carcinoma).
(B) CNB can be used as an alternative to FNA for thyroid nodules with US–cytology discordance in previous FNA.
In a meta-analysis by Suh et al. (95), both CNB and FNA showed high specificity (99% vs. 100%) for the diagnosis of thyroid malignancy. However, CNB demonstrated a significantly lower pooled proportion of non-diagnostic (5.5%, p < 0.001) and inconclusive (8.0%, p < 0.001) results than FNA (22.6% and 40.2%, respectively) and higher sensitivity (91% vs. 74%, p = 0.053). The area under the curve (0.99 vs. 0.94) was also higher in the CNB studies than in the FNA studies. In addition, the frequency of non-diagnostic CNB results was significantly higher for studies published before 2013 than for those published in 2013 or later (10.9% vs. 0.9%, p < 0.001). This difference was explained by the development of devices and technical advances during recent years.
False-positive results have not been reported in CNB results with Bethesda category 6 and have rarely been reported with Bethesda category 5 (224796). A recent large cohort study (97) of 676 surgically diagnosed cases reported a false-positive rate in malignant CNB diagnosis of 2%. The rate of false-negative results is generally low, ranging from 0 to 3.8% (4172021224577979899). In large cohort studies (each including more than 500 patients), the rates of false-negative results ranged from 1% to 3% (227797). The reasons for the false-negative cases were targeting error, large nodules with heterogeneous component, and cancers with severe calcification and/or fibrosis (18223637). Therefore, to minimize false-negative results from these thyroid nodules, sampling from at least two locations is required.
Regarding the diagnostic accuracy of thyroid malignancy, a meta-analysis by Suh et al. (95) compared the results of 12 CNB studies (2122454756575969859699100) with those of 6 FNA studies (224556575996). The specificities of both CNB and FNA were very high (both 99.5%) but the sensitivity was higher for CNB (74% [95% confidence interval, 67–81%] vs. 50% [95% confidence interval, 44–56%]).
The guidelines, including those of the NCI and previous studies, suggest that CNB is safe, well-tolerated, and associated with a low incidence of complications when performed by experienced operators (182132). The reported complication rate ranges from 0 to 4.1%, with the major complication rate ranging from 0 to 1.9% (19454750101). Because CNB is performed under real-time US guidance, serious complications seem to be rare. Nonetheless, various complications may occur, including hematoma (18212245101), hoarseness (1947), infection (98101), hemoptysis (21), edema (151822), vasovagal reaction (101), and dysphagia (101). Besides these complications, a recent large single-center study (6687 thyroid nodules of 6169 patients) found low rates of major and minor complications (4/6169 [0.06%] and 49/6169 [0.79%], respectively) and no procedure-related death or sequelae (102). To avoid complications, CNB should be performed by an expert in the field of thyroid intervention with continuous monitoring of the needle tip during the procedure. Understanding of the neck anatomy, anatomic variations, and potential complications is also required for the safe performance of CNB (103).
Pain and discomfort during or after CNB are a common problem. However, CNB using 18–22-gauge cutting needles with US guidance has allowed operators to decrease the level of pain and frequency of complications (18). Two recent studies compared the pain and tolerability of FNA and CNB (104105) and concluded that the two procedures are similar in terms of pain and tolerability.
Vascular injury, resulting from needle-induced mechanical injury, is the most common complication after thyroid CNB. Hematoma is the most common type of vascular injury, although pseudoaneurysm and arteriovenous fistula have been also reported after CNB and FNA (102106107). The incidence of vascular injury has been reported to be up to 3.9% (102108), which is similar to that of FNA (1–6.4%) (106). Edema can be associated with hematoma and pain (15182237). During the intervention of thyroid, various types of hematomas can be detected in perithyroidal, subcapsular, and intranodular locations (90) but are usually successfully managed with simple compression of the neck for between 30 and 120 minutes, with most hematomas disappearing within 1 or 2 weeks (1822). Although most hematomas are well controlled by manual compression, a few cases of uncontrolled hemorrhage have been reported after FNA that necessitated hospital admission and active intervention due to acute upper airway obstruction (109110111). For CNB, hemorrhage has been reported as a major complication (45112). Therefore, in patients taking drugs associated with a bleeding tendency, operators and physicians should carefully consider the risk-benefit ratio and withdraw those drugs before CNB. During the CNB, perithyroidal vessels, including the superior and inferior thyroid arteries, should be carefully evaluated using Doppler US. The needle tip should not be advanced across the thyroid capsule to prevent vascular injury (103). Manual compression should be performed immediately after the biopsy for 20 to 30 minutes after CNB. Patients should be educated about the possibility of delayed hematoma and how to manage the complication, although most hematomas happen during or just after CNB (1518).
Voice change, caused by injury to the recurrent laryngeal nerve, is a rare but serious complication after thyroid CNB. A voice change incidence of up to 1.9% has been reported in CNB (19), which is similar to that of FNA (0.036–0.9%) (106). Quick stretching of the nerve over the thyroid swelling and/or pressure on the nerve against the trachea could be possible mechanisms of recurrent laryngeal nerve palsy after CNB. Hemorrhage around the nerve can also cause voice problems. Recurrent laryngeal nerve palsy associated with hemorrhage is usually transient and ameliorates after the hemorrhage is spontaneously absorbed (113). Voice changes are usually transient but a case of permanent injury has been reported in CNB because of direct cutting of the recurrent laryngeal nerve through a lateral approach method (97). A transisthmic approach is recommended to prevent direct injury to the recurrent laryngeal nerve. A safe distance from the needle tip should be carefully measured before the stylet is fired and the specimen notch should be monitored (103).
Thyroid infection and/or abscess formation have been reported after CNB (98), but their incidence is very low due to various protective mechanisms, including a rich blood supply, rich lymphatic drainage, high content of iodine, and the capsule surrounding the gland. Therefore, prophylactic antibiotics are not recommended before or after CNB. However, infection at the needle puncture site or direct injury to the esophagus can cause infection (12). The puncture site should be sterilized to prevent an infection, and knowledge of US-based anatomy is necessary so as not to misdiagnose the esophageal or pharyngoesophageal diverticulum as a thyroid nodule (103114). In the case of a mass located at the posteromedial margin of the thyroid, CNB should be performed only after the operator verifies that it is not a pharyngoesophageal or esophageal diverticulum.
Cough and/or hemoptysis may be caused by direct injury to the trachea by the core needle. Although puncture of the trachea is a possible complication, there has been only one case report of hemoptysis each after CNB (21) and FNA (115). The hemoptysis spontaneously resolved and did not require hospitalization.
Extrathyroidal tissue injury, including vessel, muscle, or vertebral, may occur when the needle tip advances too far across the thyroid capsule. Tinnitus has been reported, caused by injury of the vertebral artery followed by an arteriovenous fistula (107). A safe distance from the needle tip should be carefully measured before the stylet is fired so that it is not outside the thyroid capsule. Vasovagal reaction and dysphagia are possible complications that can improve with conservative treatments (101). Tumor seeding after CNB of thyroid nodules has not been reported (116).
For safe and effective US-guided CNB, physicians should understand the broad spectrum of complications as well as how to prevent them. Knowledge of US-based thyroidal and perithyroidal anatomy including vessels and nerves is required, as well as estimation of the risk-benefit ratio of CNB, to prevent and minimize complications.
[Recommendation 11]
Core needle biopsy is safe, well-tolerated, and associated with a low incidence of complications when performed by experienced operators.
The accurate interpretation of CNB requires good knowledge of both clinical and US findings, and this information should be provided to pathologists on the request form. The request form should include patient identifiers, gender, clinical details, US findings, the biopsy site, and the number of biopsy cores.
The optimal fixation of CNB specimens is paramount. Biopsy cores should be wrapped in saline- or fixative-moistened gauze or filter paper to prevent specimen loss and tissue folding and be placed in 10% neutral buffered formalin solution as soon as possible. The fixative volume should be at least 10 times that of the tissue specimen. The duration of tissue fixation should be between at least 6 and 72 hours at room temperature (117).
Core needle biopsy mostly provides a histological diagnosis to distinguish malignant from benign nodules but it sometimes cannot give a definite diagnosis. A categorical reporting system for CNB helps to ensure effective communication between pathologists and clinicians, with less likelihood of misinterpretation of pathologic results. Thus, the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group (118) has developed a microscopic reporting system for thyroid CNB based on the six-level reporting categories of the Bethesda System for Reporting Thyroid Cytopathology (54).
Non-diagnostic thyroid nodule specimens have too few or no follicular cells for diagnosis. Inadequate sampling of the nodule includes extralesional (normal) thyroid tissue only, extrathyroidal soft tissue only, or a blood clot only. When a CNB specimen is taken only from intralesional tissue and microscopically reveals normal-appearing follicular cells, it may be difficult to determine whether the sample is representative of the nodule or inadequately biopsied from extralesional thyroid tissue. Thus, the interpretation of CNB should be based on detailed knowledge of US findings. The frequency of CNB specimen misinterpretation can be reduced if it includes the transitional area between the follicular lesion and the surrounding thyroid parenchyma.
Acellular or paucicellular specimens are generally non-diagnostic. A low cellularity of CNB specimens usually results from fibrosis, sclerosis, or calcification within the nodule. Because these microscopic findings can occur in benign and malignant thyroid lesions, pathologic diagnosis should be based on only follicular cells (119). If atypical cells are found in the paucicellular fibrotic specimen, an appropriate categorical diagnosis should be rendered instead of a non-diagnostic category (Fig. 4A). However, when a fibrotic CNB specimen shows too few benign-appearing follicular cells for a proper diagnosis, it is considered non-diagnostic.
In the pathology report of CNB, the reasons for a non-diagnostic sample should be detailed because the threshold for a non-diagnostic sample may be subjective and the clinical outcome may differ depending on the cause. Further studies should be done to determine the minimum number of benign follicular cells needed for an adequate CNB.
The benign category includes any benign thyroidal disease as well as benign nonthyroidal lesions. Nodular hyperplasia is the most common benign follicular lesion. Other benign diseases include chronic lymphocytic thyroiditis, subacute granulomatous thyroiditis, and parathyroid lesion.
The indeterminate category can be subcategorized into three subtypes based on the cytological and architectural atypia.
A. Indeterminate Follicular Lesion with Nuclear Atypia
In this category, follicular cells show nuclear atypia, but the nuclear findings are not competent to diagnose papillary carcinoma or other malignancy.
B. Indeterminate Follicular Lesion with Architectural Atypia
Architectural atypia includes microfollicular, trabecular, solid, or papillary proliferation. When a follicular proliferative lesion shows architectural atypia on the CNB specimen without nuclear atypia but does not include fibrous capsule or adjacent normal tissue, it is not clear whether the nodule is an encapsulated neoplastic lesion or not. Thus, the Indeterminate Follicular Lesion with Architectural Atypia category is usually applied to uncertain follicular proliferative lesions without nuclear atypia.
C. Other Indeterminate Lesions
Pathologic diagnosis of a follicular neoplasm in the CNB sample should be correlated with US findings. Most follicular neoplasms are solitary round to ovoid nodules with a well-formed fibrous capsule. In the US image, the fibrous capsule can be seen as a hypoechoic halo around the nodule. The follicular neoplasm is microscopically diagnosed in CNB when the specimen shows follicular proliferation and a thick fibrous capsule without nuclear atypia (Fig. 4B) (49). The follicular growth patterns can be microfollicular, normofollicular, macrofollicular, and trabecular. Therefore, follicular lesions with more distinct growth patterns can be diagnosed as follicular neoplasm in CNB specimens than in FNA because the latter diagnosis for follicular neoplasm is limited to microfollicular or trabecular proliferative lesions. Accordingly, the diagnostic rate of follicular neoplasm is higher in CNB than in FNA (49). However, a subset of follicular neoplasms, such as macrofollicular and normofollicular proliferative lesions, can be interpreted as belonging to the benign category when the fibrous tumor capsule is not included in the CNB specimen. Although follicular neoplasm with nuclear atypia raises the possibility of papillary carcinoma, it should be categorized as a subset of follicular neoplasm if the nuclear atypia is not sufficient for a diagnosis of papillary carcinoma (49120).
The "suspicious for malignancy" diagnosis is applied to any lesion with suspicious but insufficient histologic findings for the diagnosis of malignancy. The diagnostic rate of this category is significantly lower in CNB than in FNA because most thyroid cancers are easily diagnosed on histologic slides (73).
Papillary carcinoma is the most common thyroid cancer (Fig. 4C). Other malignant thyroid cancers include poorly differentiated carcinoma, undifferentiated carcinoma, medullary carcinoma, lymphoma, metastatic carcinoma, and rare sarcomas. Follicular carcinoma cannot be histologically diagnosed on CNB specimens because the morphologies of follicular adenoma and carcinoma overlap and it is not possible to assess capsular or vascular invasion in CNB specimens.
The advantage of CNB over FNA is the easy ability to perform immunohistochemical or molecular testing using additional tissue sections of CNB paraffin blocks. A combination of immunohistochemical markers consisting of galectin-3, HBME-1, cytokeratin 19, or CD56 is commonly used for the diagnosis of papillary carcinoma (23121122). Panels including more than one immunostaining marker are more accurate than single markers (121). The BRAFV600E mutation is the most commonly used molecular test and is highly specific for papillary carcinoma (23). Immunohistochemistry for calcitonin can confirm the diagnosis of medullary carcinoma. Immunohistochemistry is mandatory for the diagnosis of lymphoma and should be performed on any CNB specimen suspicious for lymphoma (48).
Lack of standardization in the CNB pathologic classification has been suggested as one of the major limitations of CNB (18123). In FNA, the Bethesda System for Reporting Thyroid Cytopathology has been widely adopted (54). However, the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group recently suggested a diagnostic category for the histologic examination of CNB samples. Standardized thyroid FNA and CNB results fall within one of six diagnostic categories based on the category scheme of the Bethesda system. However, the proportions of diagnoses and the rates of malignancy for each diagnostic category may differ between FNA and CNB. In the future, it will be necessary to estimate the malignant risk of each category of the new CNB pathology classification.
The combination of molecular tests with CNB results is also challenging. The added value of molecular tests to CNB is insufficient and debatable (232472). The technique used for biopsy specimen preparation is critical to the reliability of the immunohistochemistry results of thyroid cancer (124).
The indication for CNB is also still debatable. Many articles suggested such CNB indications as thyroid nodules with previously inconclusive FNA results, calcified thyroid nodules, or suspicious US features, or even that it should be used as a first-line diagnostic tool (151843475059). However, the evidence is still insufficient because of the retrospective design of these studies and their small patient populations. Large multicenter and/or multinational prospective studies are necessary to establish the indications for CNB of thyroid nodules.
A safe and effective CNB technique needs to be established, which would consider the type and size of the CNB needle, approach, route to nodule, number of samplings, way of cutting the nodule and adjacent parenchyma, and knowledge for minimizing procedure-related complications.
The cost of CNB may be substantially higher than that of conventional FNA in some countries (124). Therefore, cost-effective analysis according to the individual country should be performed.
Figures and Tables
Fig. 2
CNB procedure on US.
A. Insertion of core needle through isthmus. B. Measurement of distance of fire (arrows). C. Firing of stylet. Specimen notch includes nodule, nodule capsule, and small amount of normal thyroid parenchyma. D. Firing of cutting cannula. CNB = core needle biopsy, RLP = right lower pole US = ultrasound
Fig. 3
Nodule in deep posterior portion of thyroid gland.
A. Nodule is located in posterior portion near thyroid capsule. B. Stylet is manually advanced into nodule. C. Then, nodule is elevated with inserted needle. Direction of firing would be changed after adjusting stylet to adopt safer direction. D. Finally, cutting cannula is fired. CNB = core needle biopsy
Fig. 4
Representative microscopic images of core needle biopsy specimens.
A. Paucicellular fibrotic nodule with calcification shows few atypical follicular cells with nuclear atypia and can be diagnosed as papillary carcinoma. Shown at × 12.5 original magnification (left), × 100 original magnification (middle) and × 400 original magnification (right). Hematoxylin and eosin stain was used. B. Core needle biopsy specimen consists of microfollicular proliferative lesion, fibrous capsule, and surrounding normal parenchyma. In high-power view, follicular cells have no nuclear atypia. This case can be diagnosed as follicular neoplasm. Shown at × 40 original magnification (left) and × 400 original magnification (right). Hematoxylin and eosin stain was used. C. Case of classic papillary carcinoma. Specimen shows papillary proliferative lesion with typical nuclear features of papillary carcinoma. Shown at × 12.5 original magnification (left) and × 400 original magnification (right). Hematoxylin and eosin stain was used.
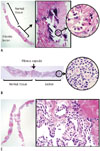
Table 1
Scope of Recommendations

Table 2
Methodology

| Category | Content |
|---|---|
| Methods used to collect/select evidence | Searches of electronic databases, including Ovid-Medline |
| Literature search procedure | Medline literature search was based on keywords provided by topic author and validated by main authors (first and corresponding authors) |
| Methods used to formulate recommendations | Modified Delphi methodology |
| Cost analysis | In most of involved thyroid centers, cost of fine-needle aspiration and core needle biopsy is similar. In specific conditions, cost is considered for biopsy tools and management decisions |
| Method of guideline validation | Internal peer review was performed by members of Korean Society of Thyroid Radiology after making draft available for 1 month at home page of Korean Society of Thyroid Radiology (http://www.thyroidimaging.kr) |
Table 3
Identifying Information and Availability

| Category | Content |
|---|---|
| Date released | 2013 (revised 2016) |
| Guideline developer(s) | Korean Society of Radiology, Korean Society of Thyroid Radiology |
| Source(s) of funding | Korean Society of Radiology provided funding and resources for these recommendations |
| Guideline committee | Committee on recommendations and task force team for thyroid core needle biopsy |
| Composition of group that authored the guideline: Dong Gyu Na, MD, PhD, Jung Hwan Baek, MD, PhD, So Lyung Jung, MD, PhD, Ji-hoon Kim, MD, PhD, Jin Yong Sung, MD, Kyu Sun Kim, MD, Jeong Hyun Lee, MD, PhD, Jung Hee Shin, MD, PhD, Yoon Jung Choi, MD, Eun Ju Ha, MD, PhD, Hyun Kyung Lim, MD, Soo Jin Kim, MD, Soo Yeon Hahn, MD, Kwang Hwi Lee, MD, Young Jun Choi, MD, Inyoung Youn, MD, Young Joong Kim, MD, Hye Shin Ahn, MD, Ji Hwa Ryu, MD, Seon Mi Baek, MD, Jung Suk Sim, MD, PhD, Chan Kwon Jung, MD, PhD, Joon Hyung Lee, MD, PhD | |
| Financial disclosures/conflicts of interest | None of guideline committee have financial disclosure or conflict of interest |
| Guideline status | This is current release of guideline |
| Guideline availability | Electronic copies: available from Korean Society of Thyroid Radiology web site (http://www.thyroidimaging.kr) |
Table 4
Summary of Consensus Statement and Recommendations

References
1. Pitman MB, Abele J, Ali SZ, Duick D, Elsheikh TM, Jeffrey RB, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:407–424.
2. Silverman JF, West RL, Finley JL, Larkin EW, Park HK, Swanson MS, et al. Fine-needle aspiration versus large-needle biopsy or cutting biopsy in evaluation of thyroid nodules. Diagn Cytopathol. 1986; 2:25–30.
3. Wang C, Vickery AL Jr, Maloof F. Needle biopsy of the thyroid. Surg Gynecol Obstet. 1976; 143:365–368.
4. Pisani T, Bononi M, Nagar C, Angelini M, Bezzi M, Vecchione A. Fine needle aspiration and core needle biopsy techniques in the diagnosis of nodular thyroid pathologies. Anticancer Res. 2000; 20:3843–3847.
5. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules--2016 Update. Endocr Pract. 2016; 22:622–639.
6. Tee YY, Lowe AJ, Brand CA, Judson RT. Fine-needle aspiration may miss a third of all malignancy in palpable thyroid nodules: a comprehensive literature review. Ann Surg. 2007; 246:714–720.
7. Wang CC, Friedman L, Kennedy GC, Wang H, Kebebew E, Steward DL, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011; 21:243–251.
8. Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. 2002; 87:4924–4927.
9. Orija IB, Piñeyro M, Biscotti C, Reddy SS, Hamrahian AH. Value of repeating a nondiagnostic thyroid fine-needle aspiration biopsy. Endocr Pract. 2007; 13:735–742.
10. Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009; 117:195–202.
11. Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007; 111:306–315.
12. Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007; 111:508–516.
13. Deveci MS, Deveci G, LiVolsi VA, Baloch ZW. Fine-needle aspiration of follicular lesions of the thyroid. Diagnosis and follow-Up. Cytojournal. 2006; 3:9.
14. Yoo C, Choi HJ, Im S, Jung JH, Min K, Kang CS, et al. Fine needle aspiration cytology of thyroid follicular neoplasm: cytohistologic correlation and accuracy. Korean J Pathol. 2013; 47:61–66.
15. Yeon JS, Baek JH, Lim HK, Ha EJ, Kim JK, Song DE, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013; 268:274–280.
16. Novoa E, Gürtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck. 2012; 34:1497–1503.
17. Liu Q, Castelli M, Gattuso P, Prinz RA. Simultaneous fine-needle aspiration and core-needle biopsy of thyroid nodules. Am Surg. 1995; 61:628–632. discussion 632-633.
18. Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012; 22:468–475.
19. Park KT, Ahn SH, Mo JH, Park YJ, Park DJ, Choi SI, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck. 2011; 33:160–165.
20. Renshaw AA, Pinnar N. Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol. 2007; 128:370–374.
21. Screaton NJ, Berman LH, Grant JW. US-guided core-needle biopsy of the thyroid gland. Radiology. 2003; 226:827–832.
22. Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim JH, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012; 22:1564–1572.
23. Crescenzi A, Guidobaldi L, Nasrollah N, Taccogna S, Cicciarella Modica DD, Turrini L, et al. Immunohistochemistry for BRAF(V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm Metab Res. 2014; 46:370–374.
24. Crescenzi A, Trimboli P, Modica DC, Taffon C, Guidobaldi L, Taccogna S, et al. Preoperative assessment of TERT promoter mutation on thyroid core needle biopsies supports diagnosis of malignancy and addresses surgical strategy. Horm Metab Res. 2016; 48:157–162.
25. Trimboli P, Guidobaldi L, Amendola S, Nasrollah N, Romanelli F, Attanasio D, et al. Galectin-3 and HBME-1 improve the accuracy of core biopsy in indeterminate thyroid nodules. Endocrine. 2016; 52:39–45.
26. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008; 247:762–770.
27. Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2015; 16:391–401.
28. Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012; 262:335–342.
29. Sung JY, Baek JH, Jung SL, Kim JH, Kim KS, Lee D, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015; 25:112–117.
30. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–395.
31. Baek JH, Na DG, Lee JH, Jung SL, Kim JH, Sung JY, et al. Core needle biopsy of thyroid nodules: consensus statement and recommendations. J Korean Soc Ultrasound Med. 2013; 32:95–102.
32. Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008; 5:6.
33. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81:Suppl 1. 1–122.
34. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
35. Samir AE, Vij A, Seale MK, Desai G, Halpern E, Faquin WC, et al. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid. 2012; 22:461–467.
36. Yi KS, Kim JH, Na DG, Seo H, Min HS, Won JK, et al. Usefulness of core needle biopsy for thyroid nodules with macrocalcifications: comparison with fine-needle aspiration. Thyroid. 2015; 25:657–664.
37. Ha EJ, Baek JH, Lee JH, Kim JK, Kim JK, Lim HK, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol. 2014; 24:1403–1409.
38. Lee KH, Shin JH, Oh YL, Hahn SY. Atypia of undetermined significance in thyroid fine-needle aspiration cytology: prediction of malignancy by US and comparison of methods for further management. Ann Surg Oncol. 2014; 21:2326–2331.
39. Na DG, Min HS, Lee H, Won JK, Seo HB, Kim JH. Role of core needle biopsy in the management of atypia/follicular lesion of undetermined significance thyroid nodules: comparison with repeat fine-needle aspiration in subcategory nodules. Eur Thyroid J. 2015; 4:189–196.
40. Buxey K, Serpell J. Importance of core biopsy in the diagnosis of thyroid lymphoma. ANZ J Surg. 2012; 82:90.
41. Kwak JY, Kim EK, Ko KH, Yang WI, Kim MJ, Son EJ, et al. Primary thyroid lymphoma: role of ultrasound-guided needle biopsy. J Ultrasound Med. 2007; 26:1761–1765.
42. Nam M, Shin JH, Han BK, Ko EY, Ko ES, Hahn SY, et al. Thyroid lymphoma: correlation of radiologic and pathologic features. J Ultrasound Med. 2012; 31:589–594.
43. Ha EJ, Baek JH, Na DG, Kim JH, Kim JK, Min HS, et al. The role of core needle biopsy and its impact on surgical management in patients with medullary thyroid cancer: clinical experience at 3 medical institutions. AJNR Am J Neuroradiol. 2015; 36:1512–1517.
44. Del Cura JL, Zabala R, Corta I. [US-guided interventional procedures: what a radiologist needs to know]. Radiologia. 2010; 52:198–207.
45. Harvey JN, Parker D, De P, Shrimali RK, Otter M. Sonographically guided core biopsy in the assessment of thyroid nodules. J Clin Ultrasound. 2005; 33:57–62.
46. Nasrollah N, Trimboli P, Guidobaldi L, Cicciarella Modica DD, Ventura C, Ramacciato G, et al. Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate. A new sampling technique. Endocrine. 2013; 43:659–665.
47. Zhang M, Zhang Y, Fu S, Lv F, Tang J. Thyroid nodules with suspicious ultrasound findings: the role of ultrasound-guided core needle biopsy. Clin Imaging. 2014; 38:434–438.
48. Hahn SY, Shin JH, Han BK, Ko EY, Ko ES. Ultrasonography-guided core needle biopsy for the thyroid nodule: does the procedure hold any benefit for the diagnosis when fine-needle aspiration cytology analysis shows inconclusive results? Br J Radiol. 2013; 86:20130007.
49. Min HS, Kim JH, Ryoo I, Jung SL, Jung CK. The role of core needle biopsy in the preoperative diagnosis of follicular neoplasm of the thyroid. APMIS. 2014; 122:993–1000.
50. Yoon RG, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Diagnosis of thyroid follicular neoplasm: fine-needle aspiration versus core-needle biopsy. Thyroid. 2014; 24:1612–1617.
51. López JI, Zabala R, Del Cura JL. Histological diagnosis of thyroid disease using ultrasound-guided core biopsies. Eur Thyroid J. 2013; 2:29–36.
52. Kwok A, Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol. 2009; 104:3085–3097. quiz 3098.
53. Na DG, Kim DS, Kim SJ, Ryoo JW, Jung SL. Thyroid nodules with isolated macrocalcification: malignancy risk and diagnostic efficacy of fine-needle aspiration and core needle biopsy. Ultrasonography. 2016; 35:212–219.
54. Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009; 19:1159–1165.
55. Richards ML, Bohnenblust E, Sirinek K, Bingener J. Nondiagnostic thyroid fine-needle aspiration biopsies are no longer a dilemma. Am J Surg. 2008; 196:398–402.
56. Choi SH, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol. 2014; 24:2819–2826.
57. Lee SH, Kim MH, Bae JS, Lim DJ, Jung SL, Jung CK. Clinical outcomes in patients with non-diagnostic thyroid fine needle aspiration cytology: usefulness of the thyroid core needle biopsy. Ann Surg Oncol. 2014; 21:1870–1877.
58. Chen BT, Jain AB, Dagis A, Chu P, Vora L, Maghami E, et al. Comparison of the efficacy and safety of ultrasound-guided core needle biopsy versus fine-needle aspiration for evaluating thyroid nodules. Endocr Pract. 2015; 21:128–135.
59. Trimboli P, Nasrollah N, Guidobaldi L, Taccogna S, Cicciarella Modica DD, Amendola S, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. 2014; 12:61.
60. Yunker WK, Hassan SF, Ferrell LB, Hicks MJ, Giannoni CM, Wesson DE, et al. Needle core biopsy in the diagnosis of pediatric thyroid neoplasms: a single institution retrospective review. Pediatr Surg Int. 2013; 29:437–443.
61. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010; 16:Suppl 1. 1–43.
62. Suh CH, Baek JH, Kim KW, Sung TY, Kim TY, Song DE, et al. The role of core-needle biopsy for thyroid nodules with initially nondiagnostic fine-needle aspiration results: a systematic review and meta-analysis. Endocr Pract. 2016; 22:679–688.
63. Pyo JS, Sohn JH, Kang G. Core needle biopsy is a more conclusive follow-up method than repeat fine needle aspiration for thyroid nodules with initially inconclusive results: a systematic review and meta-analysis. J Pathol Transl Med. 2016; 50:217–224.
64. Wolinski K, Stangierski A, Ruchala M. Comparison of diagnostic yield of core-needle and fine-needle aspiration biopsies of thyroid lesions: systematic review and metaanalysis. Eur Radiol. 2016; 04. 18. [Epub]. DOI: 10.1007/s00330-016-4356-9.
65. Cao H, Kao RH, Hsieh MC. Comparison of core-needle biopsy and fine-needle aspiration in screening for thyroid malignancy: a systematic review and meta-analysis. Curr Med Res Opin. 2016; 32:1291–1301.
66. Heller KS. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS): is there a correct answer? Thyroid. 2014; 24:787–788.
67. Ho AS, Sarti EE, Jain KS, Wang H, Nixon IJ, Shaha AR, et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS). Thyroid. 2014; 24:832–839.
68. Yoon JH, Kwak JY, Kim EK, Moon HJ, Kim MJ, Kim JY, et al. How to approach thyroid nodules with indeterminate cytology. Ann Surg Oncol. 2010; 17:2147–2155.
69. Choi YJ, Baek JH, Ha EJ, Lim HK, Lee JH, Kim JK, et al. Differences in risk of malignancy and management recommendations in subcategories of thyroid nodules with atypia of undetermined significance or follicular lesion of undetermined significance: the role of ultrasound-guided core-needle biopsy. Thyroid. 2014; 24:494–501.
70. Brauner E, Holmes BJ, Krane JF, Nishino M, Zurakowski D, Hennessey JV, et al. Performance of the afirma gene expression classifier in Hürthle cell thyroid nodules differs from other indeterminate thyroid nodules. Thyroid. 2015; 25:789–796.
71. Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012; 367:705–715.
72. Choi SH, Baek JH, Lee JH, Choi YJ, Song DE, Chung KW, et al. Evaluation of the clinical usefulness of BRAFV600E mutation analysis of core-needle biopsy specimens in thyroid nodules with previous atypia of undetermined significance or follicular lesions of undetermined significance results. Thyroid. 2015; 25:897–903.
73. Hakala T, Kholová I, Sand J, Saaristo R, Kellokumpu-Lehtinen P. A core needle biopsy provides more malignancy-specific results than fine-needle aspiration biopsy in thyroid nodules suspicious for malignancy. J Clin Pathol. 2013; 66:1046–1050.
74. Schreiner AM, Yang GC. Adenomatoid nodules are the main cause for discrepant histology in 234 thyroid fine-needle aspirates reported as follicular neoplasm. Diagn Cytopathol. 2012; 40:375–379.
75. Han S, Shin JH, Hahn SY, Oh YL. Modified core biopsy technique to increase diagnostic yields for well-circumscribed indeterminate thyroid nodules: a retrospective analysis. AJNR Am J Neuroradiol. 2016; 37:1155–1159.
76. Choi SH, Han KH, Yoon JH, Moon HJ, Son EJ, Youk JH, et al. Factors affecting inadequate sampling of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Clin Endocrinol (Oxf). 2011; 74:776–782.
77. Suh CH, Baek JH, Lee JH, Choi YJ, Kim JK, Sung TY, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. 2016; 26:395–403.
78. Avula S, Daneman A, Navarro OM, Moineddin R, Urbach S, Daneman D. Incidental thyroid abnormalities identified on neck US for non-thyroid disorders. Pediatr Radiol. 2010; 40:1774–1780.
79. Sharma A, Jasim S, Reading CC, Ristow KM, Villasboas Bisneto JC, Habermann TM, et al. Clinical presentation and diagnostic challenges of thyroid lymphoma: a cohort study. Thyroid. 2016; 26:1061–1067.
80. Ha EJ, Baek JH, Lee JH, Kim JK, Song DE, Kim WB, et al. Core needle biopsy could reduce diagnostic surgery in patients with anaplastic thyroid cancer or thyroid lymphoma. Eur Radiol. 2016; 26:1031–1036.
81. Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, Moon HJ. How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol. 2009; 19:1923–1931.
82. Chernyavsky VS, Shanker BA, Davidov T, Crystal JS, Eng O, Ibrahim K, et al. Is one benign fine needle aspiration enough? Ann Surg Oncol. 2012; 19:1472–1476.
83. Shin JH, Han BK, Ko K, Choe YH, Oh YL. Value of repeat ultrasound-guided fine-needle aspiration in nodules with benign cytological diagnosis. Acta Radiol. 2006; 47:469–473.
84. Ha EJ, Baek JH, Lee JH, Lee HY, Song DE, Kim JK, et al. A focal marked hypoechogenicity within an isoechoic thyroid nodule: is it a focal malignancy or not? Acta Radiol. 2015; 56:814–819.
85. Ha EJ, Baek JH, Lee JH, Song DE, Kim JK, Shong YK, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid. 2013; 23:703–708.
86. Ko MS, Jeong KS, Shong YK, Gong GY, Baek JH, Lee JH. Collapsing benign cystic nodules of the thyroid gland: sonographic differentiation from papillary thyroid carcinoma. AJNR Am J Neuroradiol. 2012; 33:124–127.
87. Koo JH, Shin JH, Han BK, Ko EY, Kang SS. Cystic thyroid nodules after aspiration mimicking malignancy: sonographic characteristics. J Ultrasound Med. 2010; 29:1415–1421.
88. Sohn YM, Kim EK, Moon HJ, Kim SJ, Kwak JY. Suspiciously malignant findings on ultrasound after fine needle aspiration biopsy in a thyroid nodule with initially benign ultrasound and cytologic result: to repeat or to follow-up. Clin Imaging. 2011; 35:470–475.
89. Park NH, Kim DW, Park HJ, Lee EJ, Park JS, Park SI, et al. Thyroid cysts treated with ethanol ablation can mimic malignancy during sonographic follow-up. J Clin Ultrasound. 2011; 39:441–446.
90. Lee HY, Baek JH, Ha EJ, Park JW, Lee JH, Song DE, et al. Malignant-looking thyroid nodules with size reduction: core needle biopsy results. Ultrasonography. 2016; 35:327–334.
91. Choi WJ, Baek JH, Ha EJ, Choi YJ, Hong MJ, Song DE, et al. The ultrasonography features of hyalinizing trabecular tumor of the thyroid gland and the role of fine needle aspiration cytology and core needle biopsy in its diagnosis. Acta Radiol. 2015; 56:1113–1118.
92. Lee S, Han BK, Ko EY, Oh YL, Choe JH, Shin JH. The ultrasonography features of hyalinizing trabecular tumor of the thyroid are more consistent with its benign behavior than cytology or frozen section readings. Thyroid. 2011; 21:253–259.
93. Jang H, Park CK, Son EJ, Kim EK, Kwak JY, Moon HJ, et al. Hyalinizing trabecular tumor of the thyroid: diagnosis of a rare tumor using ultrasonography, cytology, and intraoperative frozen sections. Ultrasonography. 2016; 35:131–139.
94. Carney JA. Hyalinizing trabecular tumors of the thyroid gland: quadruply described but not by the discoverer. Am J Surg Pathol. 2008; 32:622–634.
95. Suh CH, Baek JH, Lee JH, Choi YJ, Kim KW, Lee J, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine. 2016; 54:315–328.
96. Zhang S, Ivanovic M, Nemcek AA Jr, Defrias DV, Lucas E, Nayar R. Thin core needle biopsy crush preparations in conjunction with fine-needle aspiration for the evaluation of thyroid nodules: a complementary approach. Cancer. 2008; 114:512–518.
97. Paja M, del Cura JL, Zabala R, Corta I, Lizarraga A, Oleaga A, et al. Ultrasound-guided core-needle biopsy in thyroid nodules. A study of 676 consecutive cases with surgical correlation. Eur Radiol. 2016; 26:1–8.
98. Quinn SF, Nelson HA, Demlow TA. Thyroid biopsies: fine-needle aspiration biopsy versus spring-activated core biopsy needle in 102 patients. J Vasc Interv Radiol. 1994; 5:619–623.
99. Taki S, Kakuda K, Kakuma K, Annen Y, Katada S, Yamashita R, et al. Thyroid nodules: evaluation with US-guided core biopsy with an automated biopsy gun. Radiology. 1997; 202:874–877.
100. Yoo WS, Choi HS, Cho SW, Moon JH, Kim KW, Park HJ, et al. The role of ultrasound findings in the management of thyroid nodules with atypia or follicular lesions of undetermined significance. Clin Endocrinol (Oxf). 2014; 80:735–742.
101. Khoo TK, Baker CH, Hallanger-Johnson J, Tom AM, Grant CS, Reading CC, et al. Comparison of ultrasound-guided fine-needle aspiration biopsy with core-needle biopsy in the evaluation of thyroid nodules. Endocr Pract. 2008; 14:426–431.
102. Ha EJ, Baek JH, Lee JH, Kim JK, Choi YJ, Sung TY, et al. Complications following US-guided core-needle biopsy for thyroid lesions: a retrospective study of 6,169 consecutive patients with 6,687 thyroid nodules. Eur Radiol. 2016; 06. 16. [Epub]. DOI: 10.1007/s00330-016-4461-9.
103. Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015; 16:749–766.
104. Stangierski A, Wolinski K, Martin K, Leitgeber O, Ruchala M. Core needle biopsy of thyroid nodules-evaluation of diagnostic utility and pain experience. Neuro Endocrinol Lett. 2013; 34:798–801.
105. Nasrollah N, Trimboli P, Rossi F, Amendola S, Guidobaldi L, Ventura C, et al. Patient's comfort with and tolerability of thyroid core needle biopsy. Endocrine. 2014; 45:79–83.
106. Polyzos SA, Anastasilakis AD. Clinical complications following thyroid fine-needle biopsy: a systematic review. Clin Endocrinol (Oxf). 2009; 71:157–165.
107. Bergeron M, Beaudoin D. Simple core-needle biopsy for thyroid nodule, complicated tinnitus. Eur Thyroid J. 2014; 3:130–133.
108. Karstrup S, Balslev E, Juul N, Eskildsen PC, Baumbach L. US-guided fine needle aspiration versus coarse needle biopsy of thyroid nodules. Eur J Ultrasound. 2001; 13:1–5.
109. Hor T, Lahiri SW. Bilateral thyroid hematomas after fine-needle aspiration causing acute airway obstruction. Thyroid. 2008; 18:567–569.
110. Roh JL. Intrathyroid hemorrhage and acute upper airway obstruction after fine needle aspiration of the thyroid gland. Laryngoscope. 2006; 116:154–156.
111. Noordzij JP, Goto MM. Airway compromise caused by hematoma after thyroid fine-needle aspiration. Am J Otolaryngol. 2005; 26:398–399.
112. Kakiuchi Y, Idota N, Nakamura M, Ikegaya H. A fatal case of cervical hemorrhage after fine needle aspiration and core needle biopsy of the thyroid gland. Am J Forensic Med Pathol. 2015; 36:207–209.
113. Tomoda C, Takamura Y, Ito Y, Miya A, Miyauchi A. Transient vocal cord paralysis after fine-needle aspiration biopsy of thyroid tumor. Thyroid. 2006; 16:697–699.
114. Choi SH, Kim EK, Kim SJ, Kwak JY. Thyroid ultrasonography: pitfalls and techniques. Korean J Radiol. 2014; 15:267–276.
115. Silverman JF, West RL, Larkin EW, Park HK, Finley JL, Swanson MS, et al. The role of fine-needle aspiration biopsy in the rapid diagnosis and management of thyroid neoplasm. Cancer. 1986; 57:1164–1170.
116. Shah KS, Ethunandan M. Tumour seeding after fine-needle aspiration and core biopsy of the head and neck--a systematic review. Br J Oral Maxillofac Surg. 2016; 54:260–265.
117. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795.
118. Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015; 49:288–299.
119. Khoo ML, Asa SL, Witterick IJ, Freeman JL. Thyroid calcification and its association with thyroid carcinoma. Head Neck. 2002; 24:651–655.
120. Ustun B, Chhieng D, Van Dyke A, Carling T, Holt E, Udelsman R, et al. Risk stratification in follicular neoplasm: a cytological assessment using the modified Bethesda classification. Cancer Cytopathol. 2014; 122:536–545.
121. Alshenawy HA. Utility of immunohistochemical markers in diagnosis of follicular cell derived thyroid lesions. Pathol Oncol Res. 2014; 20:819–828.
122. El Demellawy D, Nasr AL, Babay S, Alowami S. Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathol Res Pract. 2009; 205:303–309.
123. Yoon JH, Kim EK, Kwak JY, Moon HJ. Effectiveness and limitations of core needle biopsy in the diagnosis of thyroid nodules: review of current literature. J Pathol Transl Med. 2015; 49:230–235.
124. Trimboli P, Crescenzi A. Thyroid core needle biopsy: taking stock of the situation. Endocrine. 2015; 48:779–785.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download