Abstract
Stenotrophomonas maltophilia (S. maltophilia) is a rare, but globally emerging gram-negative multiple-drug-resistant organism usually found in a nosocomial setting in immunocompromised patients. To our best knowledge, computed tomography (CT) features of community-acquired S. maltophilia pneumonia have not been previously reported in an immunocompetent patient. Herein, we presented the CT findings of a previous healthy 56-year-old male with S. maltophilia pneumonia.
Stenotrophomonas maltophilia (S. maltophilia) is an environmental, globally emerging gram-negative pathogen most commonly associated with respiratory tract infection in humans (1). S. maltophilia mainly causes nosocomial infections in immunocompromised patients. However, community-acquired S. maltophilia infection has been occasionally reported in patients with bacteremia, ocular infection, respiratory tract infection, cellulitis, urinary tract infection, and wound infection (2). Commonly, patients with S. maltophilia infection have some form of comorbidity such as chronic obstructive pulmonary disease, central venous catheter use, malignancy, trauma, prior hospitalization, HIV infection, or other forms of immune suppression (1).
To our best knowledge, only two case reports have described computed tomography (CT) features of S. maltophilia pneumonia in immunocompromised patients. However, the CT features of community-acquired S. maltophilia pneumonia in immunocompetent patients remain unclear. Herein, we reported a case of community-acquired S. maltophilia pneumonia in an immunocompetent patient, with particular emphasis on the CT findings. The review of this report was exempted by the Institutional Review Board of our institution.
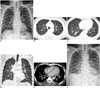
A 56-year-old man presented with a 4-day history of febrile sensation. The physical examination was unremarkable apart from a fever (39.0℃). He had been prescribed medication for hypertension; otherwise, there was no known history of malignancy or chronic disease. On admission, he had a high erythrocyte sedimentation rate (77 mm/h) and a high C-reactive protein level (26 mg/L). The white blood cell count and absolute neutrophil count were both normal (5.14 x 103/µL, 2560/µL). The initial septic work-up, which included sputum bacterial culture, acid fast bacilli smear and culture, bacterial and fungal blood cultures, and a nasal swab for respiratory viral markers, was negative. The patient was negative for HIV infection. An initial chest radiograph showed increased interstitial markings in both lungs, with a suspicion of interstitial pneumonia or interstitial pulmonary edema (Fig. 1A). Chest CT demonstrated a smooth thickening of the interlobular septae and peribronchovascular bundle with multifocal ground-glass opacities and ill-defined nodules in both lungs. A small amount of bilateral pleural effusion and pericardial effusion was present (Fig. 1B-E). The initial radiologic impression was atypical pneumonia, including viral pneumonia. Ceftriaxone was initiated as an empirical treatment, with Gemifloxacin for atypical pneumonia. The follow-up chest radiograph showed progression of bilateral ground-glass opacities and increased interstitial markings in both lungs, with increased bilateral pleural effusion (Fig. 1F). The patient underwent bronchoscopy, and subsequent culture of the bronchoalveolar lavage fluid demonstrated the presence of S. maltophilia, which was sensitive to Levofloxacin; hence, the antibiotic was switched to Levofloxacin. The patient became afebrile and further follow-up chest radiograph showed decreased bilateral ground-glass opacities and pleural effusion. Finally, the patient was discharged from the hospital.
Stenotrophomonas maltophilia is a waterborne aerobic, gram-negative multiple-drug-resistant organism, most commonly associated with respiratory tract infection in humans (1). It has emerged globally in recent years as a nosocomial infection (3). Although S. maltophilia is not a highly virulent pathogen, it is an important nosocomial pathogen associated with a high rate of mortality (14–69%) in patients with bacteremia (45). The incidence of S. maltophilia as a hospital-acquired infection is increasing, particularly in immunocompromised patients. In addition, S. maltophilia is an important emerging pathogen in cystic fibrosis patients (67). However, the clinical relevance of the increased prevalence of S. maltophilia in cystic fibrosis is unclear (8). Infections associated with S. maltophilia include respiratory tract infection, bacteremia, eye infection, endocarditis, biliary sepsis, infection of bones and joints and urinary tract infection, and also meningitis (1). In nosocomial settings, S. maltophilia has been isolated from the suction system of dental chair units, tap water, contaminated endoscopes and central venous catheters in patients with neutropenia (19).
A few reports describe S. maltophilia associated with community-acquired infection. Community-acquired S. maltophilia infections have been reported in children and adults with bacteremia, ocular infection, respiratory tract infection, cellulitis, urinary tract infection, and wound infections (2). Most patients with S. maltophilia infections have comorbidities such as malignancy, lung diseases, or an immunocompromised state (1). S. maltophilia can grow in potable water distribution systems, presenting a possible risk of infection for immunocompromised individuals (1). A previous study (10), also identified sink drains, faucets, water, and sponges as environmental sources of colonized and noncolonized S. maltophilia in the homes of cystic fibrosis patients.
To our best knowledge, only two case reports of three patients have described the CT features of S. maltophilia pneumonia. Case 1 showed CT findings of diffuse bilateral multifocal consolidation and ground-glass opacities with small centrilobular nodules in a child who had undergone bone marrow transplantation (11); Case 2 presented the CT findings of bilateral patchy ground-glass opacities without zonal predominance in a neutropenic patient with diffuse large B-cell lymphoma; and Case 3 was a neutropenic patient with advanced esophageal cancer. CT showed bilateral ground-glass opacities, consolidation, and numerous centrilobular nodules with cylindrical bronchiectasis and bronchial wall thickening without zonal predominance (12). Among these three cases, the most consistent finding of S. maltophilia pneumonia in immunocompromised patients was diffuse ground-glass opacities without zonal predominance. However, in our case, the CT findings differed from the previous reports and included interstitial thickening and ill-defined nodules with zonal predominance. These findings may resemble interstitial pneumonia, caused by Mycoplasma pneumoniae and viruses. From our experience, the CT findings of S. maltophilia pneumonia are nonspecific and the final diagnosis is still based on microbiology.
In summary, we presented the CT findings of S. maltophilia pneumonia in an immunocompetent patient. In immunocompromised patients, the CT findings of S. maltophilia pneumonia are diffuse ground-glass opacities without zonal predominance. However, in immunocompetent patients, the CT findings differ, showing interstitial thickening and ill-defined nodules with zonal predominance. Additional studies of the CT findings of S. maltophilia pneumonia are required for a better understanding of this globally emerging pathogen.
Figures and Tables
Fig. 1
56-year-old man presented with 4-day history of febrile sensation.
A. Initial chest radiograph shows increased interstitial markings in both lungs. B. Chest CT with lung window setting shows diffuse thickening of interlobular septae and ill-defined small nodules (arrowheads) in both upper lobes. C. In lower lung zones, CT scan shows diffuse smooth thickening of interlobular septae and patchy ground-glass opacities (arrows). D. On coronal scan, ill-defined small nodules (arrowheads) are predominantly seen in upper lung zones. E. Mediastinal window CT image shows small bilateral pleural effusion (arrowheads), pericardial effusion (white arrows), and thickening of peribronchovascular bundles. F. Follow-up chest radiograph shows ill-defined ground glass opacities in both parahilar areas and increased interstitial markings with developing bilateral pleural effusion.
References
1. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012; 25:2–41.
2. Falagas ME, Kastoris AC, Vouloumanou EK, Dimopoulos G. Community-acquired Stenotrophomonas maltophilia infections: a systematic review. Eur J Clin Microbiol Infect Dis. 2009; 28:719–730.
3. Lai CH, Chi CY, Chen HP, Chen TL, Lai CJ, Fung CP, et al. Clinical characteristics and prognostic factors of patients with Stenotrophomonas maltophilia bacteremia. J Microbiol Immunol Infect. 2004; 37:350–358.
4. Jang TN, Wang FD, Wang LS, Liu CY, Liu IM. Xanthomonas maltophilia bacteremia: an analysis of 32 cases. J Formos Med Assoc. 1992; 91:1170–1176.
5. Victor MA, Arpi M, Bruun B, Jønsson V, Hansen MM. Xanthomonas maltophilia bacteremia in immunocompromised hematological patients. Scand J Infect Dis. 1994; 26:163–170.
6. Denton M, Todd NJ, Littlewood JM. Role of anti-pseudomonal antibiotics in the emergence of Stenotrophomonas maltophilia in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1996; 15:402–405.
7. Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998; 27:158–163.
8. Goss CH, Otto K, Aitken ML, Rubenfeld GD. Detecting Stenotrophomonas maltophilia does not reduce survival of patients with cystic fibrosis. Am J Respir Crit Care Med. 2002; 166:356–361.
9. Sakhnini E, Weissmann A, Oren I. Fulminant Stenotrophomonas maltophilia soft tissue infection in immunocompromised patients: an outbreak transmitted via tap water. Am J Med Sci. 2002; 323:269–272.
10. Denton M, Todd NJ, Kerr KG, Hawkey PM, Littlewood JM. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol. 1998; 36:1953–1958.
11. Gasparetto EL, Bertholdo DB, Davaus T, Marchiori E, Escuissato DL. Stenotrophomonas maltophilia pneumonia after bone marrow transplantation: case report with emphasis on the high-resolution CT findings. Br J Radiol. 2007; 80:e19–e20.
12. Kassel TW, Ryan LK, Li A. Computed tomography features of Stenotrophomonas maltophilia pneumonia in patients with neutropenic fever: report of two cases. Multidiscip Respir Med. 2013; 8:14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download