Abstract
A 39-year-old female patient presented to our hospital with epigastric pain lasting for two months. Laboratory results showed impaired glucose tolerance. Ultrasonography of the patient showed a hypoechoic, diffusely enlarged pancreas. CT revealed a large pancreas, with multiple calcifications. On MRI, a diffusely enlarged pancreas was seen hypointense on both T1- and T2-weighted images with heterogeneous enhancement after gadolinium administration. A biopsy of the pancreas revealed primary amyloidosis of islet cells. Decreased signal on T1-weighted images without inflammation findings on CT and MRI were clues for the diagnosis.
Amyloidosis is a systemic disease that is characterized by the deposition of fibrillary proteins in different organs. Primary, secondary, and familial forms of amyloidosis are defined in the literature (1). The primary form is a plasma cell dyscrasia in which a light chain of an immunoglobulin is deposited. Secondary amyloidosis is due to chronic disease such as diabetes mellitus (DM), rheumatoid arthritis, and sarcoidosis. Amyloid is produced from serum amyloid A (SAA), which is a acute-phase protein (2). The familial form is a group of autosomal-dominant disease in which a mutant protein is produced (1). Although amyloidosis frequently affects multiple organs, localized forms were also reported (1). The histopathological features of pancreatic amyloidosis have been well defined in the literature; however, radiologic findings were rarely reported. In this report we present imaging findings of a patient with primary amyloidosis of pancreatic islet cells.
A 39-year-old female patient was admitted to hospital with epigastric pain which was lasting for two months. She had no remarkable history except for her mother having DM. Moreover, a physical examination revealed epigastric tenderness on palpation, and laboratory examinations revealed no important findings. Endoscopy revealed edematous and fragile papilla of Vater, and a polyp was seen on the 2nd part of duodenum. A biopsy yielded a hyperplastic polyp, and a biopsy from the papilla was performed due to the imaging findings on endoscopy with suspicion of an ampulla tumor; however, biopsy specimens yielded no malignant cell.
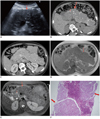
Ultrasonography (US) of the patient showed a diffusely enlarged hypoechoic pancreas with punctate hyperechogenities representing calcifications (Fig. 1A). There was no altered vascularization of pancreatic parenchyma on Doppler US. Computed tomography (CT) revealed a huge pancreas, which was enlarged diffusely with multiple calcifications and punctate hyperdensities. The pancreatic duct and peripancreatic fat tissue were normal. There was no inflammation sign (Fig. 1B, C). CT of the thorax did not yield an important feature in the mediastinum and lung parenchyma. On magnetic resonance imaging (MRI), a diffusely enlarged pancreas was seen as hypointense on both T1- and T2-weighted images (Fig. 1D, E). There were hyperintense cystic nodules in pancreas parenchyma on T2-weighted images (Fig. 1E). After gadolinium administration, enhancement of the pancreas began in the early phase, and became more prominent at delayed images. Punctate nodular unenhanced areas in pancreas parenchyma were seen on delayed images. Since the patient's history revealed no sign for chronic pancreatitis and amylase levels were normal, a trucut biopsy with an 18 G needle (Bard Biopsy Systems, Tempe, AZ) was performed. According to the histopathological results, the diagnosis was islet cell primary amyloidosis of the pancreas. Only islet cells in the islands of Langerhans were stained red with congo red ink. Exocrine cells of the pancreas were spared (Fig. 1F). Fibrosis across the islet cell islands accompanied amyloidosis. The diagnosis of primary amyloidosis was established due to a color change of amyloid deposits after permanganate administration, which is not seen in secondary amyloid deposits. An oral glucose tolerance test revealed impaired glucose tolerance with increased glucose levels (160 mg/dL) during the 2nd hour after oral glucose administration, which was attributed to impaired glucose tolerance.
Imaging findings of amyloidosis in several organs represent a wide spectrum since they can vary due to the type of amyloidosis (primary or secondary) and the degree of involvement (3). Primary amyloidosis may affect visceral organs in the abdomen including the liver, spleen, pancreas, and kidneys. Also, mesentery and retroperitoneal space may be affected in amyloidosis (3).
Pancreatic involvement of amyloidosis was described in the literature mainly with histopathological findings. Due to these reports, pancreatic amyloidosis can be seen as diffuse infiltration of exocrine and endocrine pancreas. The endocrine part of pancreas is a frequent site of involvement in secondary amyloidosis and this is mainly due to the presence of type 2 DM (4).
Despite frequent reports about histopathologic findings of pancreatic amyloidosis, we could not find enough reports regarding imaging findings of pancreatic amyloidosis. Also, these reports did not state the infiltrated portion of the pancreas (endocrine or exocrine part) in amyloidosis. Among these reports, Garcia (5) reported that the pancreas was seen enlarged and hypoechoic; diffusely in amyloidosis. On CT, pancreas was seen as diffusely hypodense and accompanying calcifications seen two years later. We also observed multiple calcifications in the pancreas. Amyloid deposits were reported to have an affinity for calcium and focal, punctate calcifications have been described in different organs with amyloid deposits (3, 5, 6).
In our case, US findings were compatible with previous reports (2, 5). Diffuse hypoechogenicity of the pancreas might be due to diffuse involvement of the pancreas. However, CT findings were interesting; we did not see any disease in our records, which may enlarge the pancreas as seen in this patient. Diffuse infiltration of the pancreatic parenchyma may be seen in lymphoma, but the presence of multiple calcification and lack of accompanying lymphadenopathies excluded this entity in the differential diagnosis (7). Auto-immune pancreatitis is another entity that must be included in the differential diagnosis of diffuse pancreatic enlargement and infiltration. However auto-immune pancreatitis characteristically cause a hypodense rim around the pancreas (7). Also, increased amounts of Ig G4 is a strong indicator of autoimmune pancreatitis with appropriate imaging findings. CT findings of our patient did not show either hypodense rim around the pancreas nor other inflammatory changes. Also, calcification is rarely seen in auto-immune pancreatitis. Acute inflammatory pancreatitis also may be investigated on patients with diffuse enlargement of the pancreas. However, the absence of pancreatic duct dilatation and lack of peripancreatic fat obliteration excluded the diagnosis of acute pancreatitis, with normal amylase levels in our case. Another feature of this case was the point that amyloidosis may mimic the ampulla Vater tumor on endoscopy by disseminating from pancreas to the papilla. Cross sectional imaging is important in these situations in order to prevent misdiagnosis.
Loss of hyperintense signal of pancreas parenchyma with primary amyloidosis on T1-weighted image was reported previously (5, 8). We observed same the same finding in our case (Fig. 1D). Decreased signal intensity on T1-weighted images may be seen in diffuse pancreatitis, diffuse involvement of pancreas in tuberculosis, as well as lymphoma and autoimmune pancreatitis. Inflammatory pancreatitis, pancreatic lymphoma, and autoimmune pancreatitis may be excluded in differential diagnosis according to other imaging findings mentioned above. Increased signal intensity in pancreas and narrowing of pancreatic duct are seen in diffuse involvement of the pancreas in tuberculosis, which was absent in our case (9).
We detected cystic lesions in pancreas parenchyma on T2-weighted images. We could not find any report indicating cystic changes in primary amyloidosis of pancreas and any other organ so cystic lesions are thought to occur incidentally. However, since imaging features of primary pancreas amyloidosis are rarely mentioned in the literature, we suggest that accompanying cystic lesions in pancreatic amyloidosis may be kept in mind. According to previous reports, increased signal intensity of the pancreas with amyloidosis was reported on T2-weighted images (5, 8). In our case, we observed a signal loss in the body and an increased signal in the pancreas tail. Our biopsy specimen was obtained from the body of the pancreas and histopathologic examination obtained after a revision request revealed fibrosis. Hence, we suggest that fibrosis may accompany amyloidosis and may cause focal decrease of signal intensity in the pancreas on T2-weighted images. This feature may explain heterogeneous contrast enhancement of the pancreas with amyloidosis described in the previous report (5). Decreased signal intensity of the pancreas on T1-weighted images is more reliable finding in the diagnosis.
Though imaging methods and laboratory findings may suggest a diagnosis or exclude some diseases affecting pancreas histopathological confirmation of amyloidosis is necessary. In our case, only Langerhans island cells were affected from amyloidosis and exocrine pancreas did not show any amyloid infiltration. To the best of our knowledge, this is the first report that specifically describes imaging findings of islet cell amyloidosis. Previous reports described the pancreatic amyloidosis without discrimination of endocrine and exocrine pancreas amyloidosis.
In our patient, the absence of other chronic disease findings and increased glucose levels at the oral glucose tolerance tests led us to think that amyloid deposition may induce DM. There was no romatologic or other systemic disease according to clinical investigations and laboratory findings as a cause of amyloidosis. It was shown that islet cell amyloidosis may be a cause of type 2 DM. Islet amyloid is present in > 90% of patients with type 2 DM. Recent data, particularly from transgenic mouse studies, indicate that islet amyloidosis is a diabetogenic factor, which is both a consequence (of insulin resistance) and cause (of beta-cell failure) of type 2 DM (10). Amyloid deposition, commonly seen in the pancreas of patients with type 2 DM, is generally classified as local amyloidosis and should not be confused with systemic involvement (4).
In conclusion, pancreatic amyloidosis may be included in the differential diagnosis of diffuse pancreatic enlargement mimicking pancreatitis. Absence of laboratory findings indicating acute pancreatitis, and abnormally enlarged pancreas may be seen in pancreatic amyloidosis. Imaging features of pancreatic amyloidosis on US, CT and MRI may alert radiologists and clinicians for the probability of DM development before prominent laboratory findings and clinical features.
Figures and Tables
Fig. 1
Imaging findings and histopathology of pancreatic amyloidosis.
A. Ultrasonography shows diffuse hypoechoic, enlarged pancreas including multiple millimetric calcifications with posterior acoustic enhancement (arrow). There is no pancreatic duct dilatation. B. Axial CT image shows diffuse prominent enlargement of pancreas (arrow), with multiple hyperdense calcifications (thin arrow). C. Axial CT after contrast enhancement on venous phase shows diffuse contrast enhancement of pancreas parenchyma with absence of inflammatory changes in peripancreratic tissues. D. Normal high signal intensity of pancreas is decreased on axial T1-weighted image. Pancreas is seen diffusely hypointense (arrow). E. Axial T2-weighted image of abdomen shows diffusely enlarged pancreas (arrow), with hyperintense cystic components (thin arrow). Pancreas tail is seen more hyperintense than body with no inflammation. Also, pancreatic duct is not dilated. F. Histopathologic examination reveals amyloid deposition stained with congo red islet cells of Langerhans (arrows).
References
1. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997. 337:898–909.
2. Georgiades CS, Neyman EG, Barish MA, Fishman EK. Amyloidosis: review and CT manifestations. Radiographics. 2004. 24:405–416.
3. Urban BA, Fishman EK, Goldman SM, Scott WW Jr, Jones B, Humphrey RL, et al. CT evaluation of amyloidosis: spectrum of disease. Radiographics. 1993. 13:1295–1308.
4. Ozdemir D, Dagdelen S, Erbas T. Endocrine involvement in systemic amyloidosis. Endocr Pract. 2010. 16:1056–1063.
5. Garcia RL. Verrucose areolar hyperpigmentation of pregnancy. Arch Dermatol. 1973. 107:774.
6. Kennan NM, Evans C. Case report: hepatic and splenic calcification due to amyloid. Clin Radiol. 1991. 44:60–61.
7. Sainani N, Catalano O, Sahani D. Haaga JR, Dogra VS, Forsting M, Gilkeson RC, Ha HK, Sundaram M, editors. Pancreas. CT and MRI of the whole body. 2009. . Philadelphia: Mosby Elsevier;1599–1675.
8. Rafal RB, Jennis R, Kosovsky PA, Markisz JA. MRI of primary amyloidosis. Gastrointest Radiol. 1990. 15:199–201.
9. De Backer AI, Mortele KJ, Bomans P, De Keulenaer BL, Vanschoubroeck IJ, Kockx MM. Tuberculosis of the pancreas: MRI features. AJR Am J Roentgenol. 2005. 184:50–54.
10. Hoppener JW, Nieuwenhuis MG, Vroom TM, Ahren B, Lips CJ. Role of islet amyloid in type 2 diabetes mellitus: consequence or cause? Mol Cell Endocrinol. 2002. 197:205–212.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download