Abstract
Objective
Our goals were to determine the added value of fine-needle aspiration biopsy (FNAB)-thyroglobulin (Tg) measurements over FNAB-cytology alone for diagnosing metastatic nodes, and to determine whether the ultrasound features of lymph nodes can be used to identify lymph nodes that may benefit from FNAB-Tg measurement in patients with papillary thyroid cancer.
Materials and Methods
We retrospectively evaluated 76 surgically proven cervical lymph nodes. Twenty-nine patients were awaiting surgery and 18 patients had undergone thyroid surgery for papillary thyroid cancer. Ultrasound-guided FNAB and Tg measurements were performed and the ultrasound features were evaluated.
Results
The accuracies, sensitivities, and specificities of FNAB-cytology, FNAB-Tg, and combined FNAB-Tg/cytology were 90%, 80%, and 100%; 92%, 95%, and 90%; and 93%, 96%, and 90%, respectively. The diagnostic sensitivity of FNAB-Tg for metastatic nodes was significantly higher than that of FNAB-cytology (p = 0.011). Furthermore, combined FNAB-Tg/cytology significantly increased sensitivity (p = 0.002) and accuracy (p = 0.03) as compared with FNAB-cytology.
The preoperative diagnosis of metastatic nodes is important for determining the optimal surgical strategy in patients with papillary thyroid carcinoma (PTC). When metastatic nodes are suspected by preoperative ultrasound (US) assessment, US fine-needle aspiration biopsy (FNAB) is recommended for the histological diagnosis of metastatic nodes in patients awaiting thyroid surgery (1). Serum thyroglobulin (Tg) assays and US are routinely recommended for the surveillance of recurrences after the surgical treatment of differentiated thyroid carcinoma (1). US-FNAB is widely used for the cytological diagnosis of lymph node metastasis from PTC preoperatively, and for determining locoregional tumor recurrence after thyroid surgery.
Thyroglobulin assessment in needle washout after fine-needle aspiration of suspicious lymph nodes is useful for the early diagnosis of lymph node metastases originating from differentiated thyroid cancer, and it is more sensitive and accurate at detecting metastatic nodes than cytology, especially in cystic nodal metastases (2-10). However, it is unclear whether FNAB-Tg measurements should be routinely accompanied by US-guided FNAB cytology for the diagnosis of metastatic nodes in patients with PTC, and it is unclear whether US features of lymph nodes may help select patients who may benefit from FNAB-Tg measurements compared with FNAB-cytology alone.
We designed this retrospective study to determine the added value of FNAB-Tg measurements over FNAB-cytology alone for diagnosing metastatic nodes, and to determine whether the US features of lymph nodes in patients with PTC can be used to identify lymph nodes that may benefit from FNAB-Tg measurements compared with FNAB cytology alone.
During a 12-month period, US-FNAB-cytology and FNAB-Tg measurements of suspected metastatic nodes were performed in 161 consecutive PTC patients at two institutions (Seoul National University Hospital and the thyroid clinic at Daerim Saint Mary's Hospital). The institutional review board approved this retrospective study and waived the requirement for informed consent.
US-FNAB and Tg measurements were performed at cervical nodes suspicious for metastasis by US, in patients that underwent US for the preoperative evaluation of suspected thyroid carcinoma, or for the postoperative surveillance of tumor recurrence. Lymph nodes for FNAB-cytology and FNAB-Tg were selected based on the presence of the following US criteria: hyperechogenicity, cystic changes, calcification, abnormal vascularity, heterogeneous echogenicity, a round shape (longitudinal/transverse diameter ratio < 1.5), loss of echogenic hilum, and a lymph node with a diameter exceeding 6 mm (11-14).
Of the 161 PTC patients, a total of 76 surgically proven cervical lymph nodes found in 47 patients (47 nodes in 30 patients awaiting thyroid surgery and 29 nodes in 17 patients being followed after thyroid surgery) were included in this study. One hundred fourteen patients were excluded because they underwent surgery or surgical neck dissection. In all 47 patients, preoperative skin marking was performed for lymph nodes where FNAB was performed. Surgeons were aware of the US, cytology, and FNAB-Tg findings and removed all skin-marked lymph nodes. In postoperative patients, a second operation was performed only when FNAB-cytology or FNAB-Tg results suggested metastatic nodes. During the first operation in patients with PTC established by FNAB-cytology, surgical neck dissections were performed on a level-by-level basis based on preoperative or intraoperative clinical palpation, US features, FNAB cytology, and Tg results.
US examinations were performed by experienced radiologists in all 47 patients before surgery using a 10-12 MHz linear transducer (Acuvix, Medison, Korea; iU22, Philips Medical Systems, Bothell, WA). US examination was performed on both thyroid lobes and the neck, including all neck levels (level I to level VI) and the supraclavicular fossa. We prospectively assessed the US features of lymph nodes and determined the presence of suspected nodal metastasis using US criteria. The US features of suspected metastatic nodes were also recorded.
US-FNAB was performed on metastatic nodes determined by US criteria using a free-hand technique with a 21-23 gauge needle. Biopsy needle content was expelled onto a slide and smeared using a second slide. Slides were fixed in 95% ethanol, Papanicolaou-stained, and read by an experienced cytopathologist. Immediately after aspiration biopsy, the needle used was washed with 0.5-1 mL of normal saline solution (final volume 1 mL). These washout samples were sent for Tg assays. Serous fluid aspirates were sent directly for assaying.
Fine-needle aspiration biopsy-cytology results were categorized as inadequate, negative (reactive lymph node or some other benign lymphadenitis), or positive for metastatic papillary carcinoma. When the measured FNAB-Tg level was greater than the serum Tg level, we deemed the lymph node positive for metastasis from PTC. In six preoperative patients whose serum Tg was not measured before surgery, we used an FNAB-Tg cutoff level of 36 ng/ml for determining positivity (8). Final lymph node status was determined from histology reports of surgical lymph node specimens. FNAB-cytology and FNAB-Tg results were subdivided into two groups for surgically proven metastatic nodes: the FNAB-Tg benefit group (FNAB-Tg positive and FNAB-cytology negative) and the no FNAB-Tg benefit group (FNAB-Tg positive and FNAB-cytology positive, FNAB-Tg negative and FNAB-cytology negative, and FNAB-Tg negative and FNAB-cytology positive). We also assessed and compared the US features of these two groups (Fig. 1).
Statistical analysis was performed using SPSS-PC (Version 10.0 1999; SPSS, Chicago, IL). The sensitivities, specificities, positive predictive values, negative predictive values, and accuracies of FNAB-Tg and FNAB-cytology for predicting the presence of metastases were determined using node-by-node analysis. To assess the diagnostic accuracy of combined FNAB-cytology and FNAB-Tg, lymph node metastasis was deemed present when either FNAB-Tg or FNAB-cytology was positive. The McNemar's test was used to compare the sensitivities, specificities, and accuracies of FNAB-Tg and FNAB-cytology with respect to metastatic node detection. Patients were divided into two groups according to the presence or absence of FNAB-Tg benefit. Differences between the groups with respect to the presence of FNAB-Tg were assessed using Fischer's exact test. Differences between maximal and minimal diameters of lymph nodes in the two groups were assessed using the Student's t test. P-values < 0.05 were considered statistically significant differences.
Fifty-six (74%) of the 76 nodes were metastatic and 20 were benign lesions (reactive lymph node - 18, tuberculous lymphadenitis - 1, and remnant thyroid tissue - 1) by histology, and included central neck nodes (n = 8) and lateral neck nodes (n = 68). Preoperative serum Tg was measured in 42 patients but not in six patients who were awaiting initial thyroid surgery. Tg antibodies were measured in only 18 patients.
Tables 1 and 2 demonstrate the diagnostic accuracies of FNAB-cytology, FANB-Tg, and combined FNAB-Tg/cytology for metastatic nodes from PTC. The results of FNAB-cytology were inadequate in three, negative in 28, and positive for malignancy in 45. For 56 metastatic nodes, FNAB-cytology detected 45 (80.4%), FNAB-Tg detected 53 (94.6%), and combined FNAB-cytology/Tg detected 54 (96.4%). The sensitivities of FNAB-Tg and FNAB-cytology/Tg were significantly higher than that of FNAB-cytology alone (p = 0.011) (Table 3). Although the diagnostic accuracy of FNAB-Tg was not significantly higher than FNAB-cytology alone, that of combined FNAB-cytology/Tg was higher than FNAB-cytology (p = 0.03). In benign lymph nodes (n = 20), FNAB-Tg levels ranged from below 0.5 to 14,640 ng/ml. In metastatic lymph nodes (n = 56), FNAB-Tg levels ranged from 0.5 to above 25,000 ng/ml. Except for two false positive FNAB-Tg cases, the FNAB-Tg levels of all benign lymph nodes were below 2.0 ng/ml. Of the three false negative FNAB-Tg cases, cytology results of FNAB-cytology were negative for malignancy in two cases and positive in one. The two false positive FNAB-Tg cases included one case of remnant thyroid tissue (FNAB Tg 14,640 and serum Tg < 1.0 ng/ml) and one level IV lymph node (FNAB Tg 82 ng/ml and serum Tg 51 ng/ml). The concordance rate between FNAB-cytology and FNAB-Tg was 84% (64 of 76 nodes). There were 12 discordant cases: FNAB-Tg positive and FNAB-cytology negative in 11 lymph nodes and FNAB-Tg negative and FNAB-cytology positive in one lymph node.
Table 4 demonstrates the incidences of US criteria for surgically proven benign and metastatic nodes. Although FNAB-Tg was positive for all cystic lymph nodes, FNAB-cytology provided a false negative result for three of eleven cystic nodes. The false negative rate of FNAB-cytology was higher for cystic lymph nodes than for lymph nodes without cystic change, but this was not significant (30% and 19%, respectively; p = 0.32). No statistically significant difference was found between lymph nodes with or without FNAB-Tg benefit with respect to US features (p > 0.05), and US features did not significantly predict the FNAB-Tg benefit group (Table 5). The mean maximal and minimal diameters of nodes were not significantly different in lymph node groups with or without FNAB-Tg benefit (maximal diameter 1.03-0.59 vs. 1.00-0.47 and minimal diameter 0.38-0.09 vs. 0.47-0.21, p = 0.8 and p = 0.9, respectively). However, FNAB-Tg benefit was only found for lymph nodes with a minimal diameter of less than 5 mm, and nodes in the FNAB-Tg benefit group tended to be smaller (less than 5 mm in minimal diameter) than nodes in the no FNAB-Tg benefit group (100% vs. 68%, p = 0.09). The positive predictive value of the US size criterion (< minimal diameter of 5 mm) for FNAB-Tg benefit was 20% and its negative predictive value was 100%.
This study demonstrates that combined FNAB-Tg/cytology is more sensitive and accurate for the detection of metastatic lymph nodes from PTC than FNAB-cytology alone. US-guided FNAB-cytology is more accurate than US, CT, and MRI for the detection of metastatic cervical nodes in head and neck malignancies (15). US-guided FNAB-cytology has high sensitivity (57-98%), specificity (92.9-100%), and accuracy (89-97%) for the detection of metastatic cervical lymph nodes in general head and neck malignancies (16-20). Moreover, FNAB-cytology has a sensitivity of 55-85%, a specificity of 90-100%, and an accuracy of 73-77% for the diagnosis of metastatic cervical lymph nodes from thyroid carcinoma (8, 10, 21). In thyroid carcinoma, FNAB-Tg is more sensitive and has a higher negative predictive value than FNAB-cytology (2, 3, 22), and FNAB-Tg/cytology increased accuracy, sensitivity, and specificity (13, 14). These results are consistent with those of the present study. FNAB-Tg/cytology significantly increased sensitivity and accuracy for the detection of metastatic nodes from PTC as compared with FNAB-cytology.
Fine-needle aspiration biopsy-Tg measurements enhance the diagnostic accuracy of metastasis to nodes from differentiated thyroid carcinoma as compared with FNAB-cytology alone, and in previous studies the benefit derived ranged from 8-43% (8-10). In this study, the benefit was 14%. Therefore, routine FNAB-Tg testing may not be necessary when FNAB-cytology is performed on metastatic nodes suspected by US from the standpoint of cost-effectiveness. Moreover, although we sought to identify US features capable of predicting FNAB-Tg benefit, no US feature was identified as a statistically significant predictor for FNAB-Tg benefit. Nevertheless, FNAB-Tg benefit was found only for nodes with a minimal axial diameter of less than 5 mm. The benefit of FNAB-Tg for small lymph nodes is probably due to the fact that these nodes are relatively difficult to aspirate and difficult to sample by FNAB, and the sampled cytology results may be difficult to interpret (23). Previous studies (2, 10) have reported high false negative rates for FNAB-cytology and greater benefit for FNAB-Tg in cystic metastatic nodes. In the present study, however, the benefit of FNAB-Tg was not significantly different for cystic and non-cystic nodes, which may be explained by the fact that the majority of cystic metastatic nodes included in our study showed only partial cystic changes. Because PTC may manifest predominantly or almost entirely as a cystic nodal mass mimicking a cyst (24), FNAB-Tg may be beneficial in lymph nodes with extensive cystic changes.
Although FNAB-Tg is obviously more sensitive than FNAB-cytology, discordant FNAB-Tg negative and FNAB-cytology positive cases could account for 2% and 6% of nodes examined (8, 9), which is similar to the 1% (1 of 76) we obtained. The reason for negative FNAB-Tg and positive FNAB-cytology discordances is unclear, though it might be related to small remnant aspirated tissue in needles used for Tg measurements or to technical errors. In one of the two cases with a false positive FNAB-Tg result, high TG levels were caused by remnant thyroid tissue, which was misdiagnosed as a recurrent tumor on the operation bed. Cautious interpretation of positive FNAB-Tg results is needed for central neck nodes in postoperative patients because remnant thyroid tissue may mimic locally recurrent tumors on the operation bed by US. However, the cause of the false positive result in the other case is unclear. The presence of false positive and false negative results in FNAB-Tg indicates that it should be used with FNAB-cytology instead of replacing FNAB-cytology.
Several limitations of the present study should be considered. First, the number of patients recruited was relatively small. Second, we could not use serum Tg level as a cut-off value for determining positive FNAB-Tg in six patients that did not undergo serum Tg testing preoperatively. However, false negative FNAB-Tg results were not influenced by the relatively high absolute Tg cut-off level in patients that did not undergo serum Tg testing, because the FNAB-Tg levels were less than 1 ng/ml in all FNAB-Tg false negative cases.
In conclusion, combined FNAB-cytology/Tg is more sensitive and accurate for the diagnosis of metastatic cervical lymph nodes from PTC than FNAB-cytology alone. Although no US feature significantly predicted FNAB-Tg benefit, FNAB-Tg testing provided significant benefit over FNAB-cytology when lymph nodes have a minimal axial diameter of less than 5 mm.
Figures and Tables
Fig. 1
52-year-old man with recurrent papillary thyroid carcinoma.
US (transverse view) shows heterogeneous and hyperechoic elongated lymph node (arrow) with focal cystic change (arrowhead) in left lateral neck (level IV). Maximal and minimal diameters were 9 mm and 4 mm, respectively. Result of US-fine-needle aspiration biopsy cytology was suboptimal (mainly blood), but measured Thyroglobulin from aspirate of node was 2,640 ng/ml.
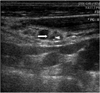
Table 1
FNAB-Tg, FNAB-Cytology, FNAB-Tg/Cytology, and Surgical Pathology Results for 76 Cervical Lymph Nodes

References
1. American thyroid association guideline. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006. 16:1–33.
2. Cignarelli M, Ambrosi A, Marino A, Lamacchia O, Campo M, Picca G, et al. Diagnostic utility of thyroglobulin detection in fine-needle aspiration of cervical cystic metastatic lymph nodes from papillary thyroid cancer with negative cytology. Thyroid. 2003. 13:1163–1167.
3. Baskin HJ. Detection of recurrent papillary thyroid carcinoma by thyroglobulin assessment in the needle washout after fine-needle aspiration of suspicious lymph nodes. Thyroid. 2004. 14:959–963.
4. Frasoldati A, Toschi E, Zini M, Flora M, Caroggio A, Dotti C, et al. Role of thyroglobulin measurement in fine-needle aspiration biopsies of cervical lymph nodes in patients with differentiated thyroid cancer. Thyroid. 1999. 9:105–111.
5. Lee MJ, Ross DS, Mueller PR, Daniels GH, Dawson SL, Simeone JF. Fine-needle biopsy of cervical lymph nodes in patients with thyroid cancer: a prospective comparison of cytopathologic and tissue marker analysis. Radiology. 1993. 187:851–854.
6. Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Elisei R, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. 1992. 74:1401–1404.
7. Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005. 90:5566–5575.
8. Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, et al. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg. 2005. 29:483–485.
9. Snozek CL, Chambers EP, Reading CC, Sebo TJ, Sistrunk JW, Singh RJ, et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab. 2007. 92:4278–4281.
10. Cunha N, Rodrigues F, Curado F, Ilheu O, Cruz C, Naidenov P, et al. Thyroglobulin detection in fine-needle aspirates of cervical lymph nodes: a technique for the diagnosis of metastatic differentiated thyroid cancer. Eur J Endocrinol. 2007. 157:101–107.
11. Ahuja AT, Chow L, Chick W, King W, Metreweli C. Metastatic cervical nodes in papillary carcinoma of the thyroid: ultrasound and histological correlation. Clin Radiol. 1995. 50:229–231.
12. Schlumberger M, Berg G, Cohen O, Duntas L, Jamar F, Jarzab B, et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol. 2004. 150:105–112.
13. Rosario PW, de Faria S, Bicalho L, Alves MF, Borges MA, Purisch S, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005. 24:1385–1389.
14. Na DG, Lim HK, Byun HS, Kim HD, Ko YH, Baek JH. Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. AJR Am J Roentgenol. 1997. 168:1311–1316.
15. van den Brekel MW, Castelijns JA, Stel HV, Golding RP, Meyer CJ, Snow GB. Modern imaging techniques and ultrasound-guided aspiration cytology for the assessment of neck node metastases: a prospective comparative study. Eur Arch Otorhinolaryngol. 1993. 250:11–17.
16. Baatenburg de Jong RJ, Rongen RJ, Verwoerd CD, van Overhagen H, Lameris JS, Knegt P. Ultrasound-guided fine-needle aspiration biopsy of neck nodes. Arch Otolaryngol Head Neck Surg. 1991. 117:402–404.
17. van den Brekel MW, Stel HV, Castelijns JA, Croll GJ, Snow GB. Lymph node staging in patients with clinically negative neck examinations by ultrasound and ultrasound-guided aspiration cytology. Am J Surg. 1991. 162:362–366.
18. Takashima S, Sone S, Nomura N, Tomiyama N, Kovayashi T, Nakamura H. Nonpalpable lymph nodes of the neck: assessment with US and US-guided fine-needle aspiration biopsy. J Clin Ultrasound. 1997. 25:283–292.
19. van den Brekel MW, Reitsma LC, Quak JJ, Smeele LE, van der Linden JC, Snow GB, et al. Sonographically guided aspiration cytology of neck nodes for selection of treatment and follow-up in patients with N0 head and neck cancer. AJNR Am J Neuroradiol. 1999. 20:1727–1731.
20. Knappe M, Louw M, Gregor RT. Ultrasonography-guided fine-needle aspiration for the assessment of cervical metastases. Arch Otolaryngol Head Neck Surg. 2000. 126:1091–1096.
21. Sutton RT, Reading CC, Charboneau JW, James EM, Grant CS, Hay ID. US-guided biopsy of neck masses in postoperative management of patients with thyroid cancer. Radiology. 1998. 168:769–772.
22. Schlumberger M, Pacini F, Wiersinga WM, Toft A, Smith JW, Sanchez Franco F, et al. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004. 151:539–548.
23. Hall TL, Layfield LJ, Philippe A, Rosenthal DL. Sources of diagnostic error in fine needle aspiration of the thyroid. Cancer. 1989. 63:718–725.
24. Ahuja A, Ng CF, King W, Metreweli C. Solitary cystic nodal metastasis from occult papillary carcinoma of the thyroid mimicking a branchial cyst: a potential pitfall. Clin Radiol. 1998. 53:61–63.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download