Horseshoe kidneys occur in 3% of the population (1). Only 123 cases of renal malignant tumors in horseshoe kidneys have been reported on the international literature up to 1998, and about 50% of these cancers were renal cell carcinomas (2). Renal cell carcinoma that originates in a horseshoe kidney is an unusual entity. In tumor-bearing horseshoe kidneys, preoperative knowledge of the localization, extent and vascular supply of the neoplasm is indispensable for performing a complete resection of the tumorous focus without sacrificing more of the functioning renal tissue than is necessary (2). Preoperative renal angiography and superselective renal artery embolization are necessary for a limited resection, which is also known as simple enucleation.
I report here on a case of renal cell carcinoma in a horseshoe kidney that was treated with preoperative superselective renal artery embolization.
CASE REPORT
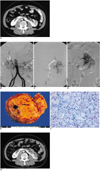
A 59-year-old man presented with liver cirrhosis and esophageal varix bleeding. A CT scan (LightSpeed QX/i, General Electric Co., Milwaukee, WI, USA) with contrast media was performed and it incidentally revealed a horseshoe kidney with a well-enhanced isthmus and a 3-cm enhancing mass in the left part (Fig. 1A). The tumor was limited to the kidney, but its extension into the renal pelvis was not definite. Digital subtraction angiography (DSA) via the right femoral artery route was performed under local anesthesia just before the embolization procedure. The DSA of the aorta showed that the horseshoe kidney was fed by a five-vessel supply that consisted of both the two normal main renal arteries, two aberrant vessels that were originating from the aorta and they entered both sides of the isthmus (Fig. 1B), and an additional vessel (Fig. 1B) that entered the left side of the isthmus and it originated from the left common iliac artery. The DSA of the left renal artery demonstrated hypervascular tumor staining that was supplied by the anterior inferior segmental branch of the left main renal artery (Fig. 1C).
Although the portion of the tumor that had invaded the collecting systems could not be completely excluded, the decision was made to perform organ-preserving surgery or simple enucleation. Preoperative superselective renal artery embolization was performed as a prerequisite for simple enucleation. Catheterization was performed via a transfemoral approach with the use of the standard coaxial technique. A 5-F end-hole catheter (Cobra catheter, Cook, Bloomington, IN, USA) was introduced over a 0.035-inch guide wire (Termo; Radifocus, Tokyo, Japan) to the left main renal artery. The feeding vessel to the tumor was catheterized with the use of a 3-F microcatheter (Renegade; Boston Scientific, Watertown, MA, USA) and it was embolized superselectively with Contour (355-500 microns, Boston Scientific International, La Garenne Colombes Cedex, France) (Fig. 1C). The contour granules were slowly and carefully injected (to prevent reflux of the particles) under fluoroscopic guidance before embolization with the use of a 0.018-inch-diameter Tornado microcoil (3 mm to 2 mm, Cook). A postembolization angiography shows a successful segmental embolization of the anterior inferior segment of the left part kidney, including the tumor (Fig. 1D).
For the patient to undergo an effective and comfortable intervention, analgesic (midazolam 2 mg, i.v.; Roche, Fontenay-sous-Bois, France) and sedative (fentanyl 60 micrograms, i.v.; Guju Pharm. Co., Hwasung, Korea) were administered just before the DSA to achieve moderate sedation.
Under general anesthesia, an anterior transverse abdominal incision was performed one day after the embolization. The tumor site was a mild brown color and it was easily identified at the left side of the horseshoe kidney because of the previous renal artery embolization. The tumor was enucleated by repeated cuts with the use of an electrosurgical generator (Valleylab Inc., Boulder, CO, USA). The parenchymal bleeding was easily controlled by suture because of the previous renal artery embolization.
Upon examination of the gross specimen, the tumor showed as a well-circumscribed, bright yellow, solid mass measuring about 3 cm at its greatest diameter (Fig. 1E). The tumor was confined to the kidney and it proved to be a renal cell carcinoma, grade 2 (Fig. 1F).
A follow-up CT scan with contrast media 33 days after the operation showed a parenchymal defect at the previous tumor site with some postoperative change (Fig. 1G).
DISCUSSION
The horseshoe kidney is probably the most common of all renal fusion anomalies (3). This anomaly consists of two distinct renal masses lying vertically on either side of the midline; the masses are connected at their respective lower poles by a parenchymatous or fibrous isthmus that crosses the midplane of the body (3).
Most of the malignant tumors arising in horseshoe kidneys are renal cell carcinomas, but transitional cell carcinomas, squamous cell carcinomas, Wilm's tumors, lymphomas, carcinoid tumors and sarcomas have also been reported (4, 5). It has been stated that the occurrence of renal cell carcinoma in horseshoe kidneys is no higher than in non-fused kidneys, but that the incidence of transitional cell carcinoma in horseshoe kidneys is higher, and this is conceivably due to the presence of chronic urinary tract infections (4).
The blood supply to the horseshoe kidney can be quite variable (3). In 30% of the cases, it consists of one renal artery for each kidney (6), but the blood supply may be atypical, with duplicate or even triplicate renal arteries supplying one or both kidneys (3). The isthmus and adjacent parenchymal masses may receive a branch from each main renal artery, or they may have their own arterial supply originating from the aorta either above or below the level of the isthmus (3). Not infrequently, this area is supplied by branches from the inferior mesenteric artery, the common or external iliac arteries, or the sacral arteries (7). In this case, the isthmus was receiving two arterial supplies that originated from the aorta at the level of the isthmus, and there was an additional arterial supply from the left common iliac artery (Fig. 1B).
An aberrant vascular supply is one of the major anatomic features in horseshoe kidneys; thus, the vascular supply cannot be easily predicted on the surgical field. Therefore, angiography is indispensable for guiding the radiologic and surgical interventions. This is especially true when preoperative renal artery embolization is necessary and a part of the organ has to be removed due to malignant disease while the maximal amount of functioning renal tissue needs to be preserved. Radical nephrectomy is the standard therapy for renal cell carcinoma (8). In cases of neoplasm in a horseshoe kidney, however, there is a place for limited resection or heminephrectomy, with special attention being paid to the abnormal arteries and the renal pelvis (8). Preoperative superselective renal artery embolization helps to prevent excessive bleeding complications during organ-preserving surgery, it allows the preservation of a maximum amount of functioning renal tissue and it enables easy detection of the tumor site via the discoloration; thus, simple enucleation is then feasible during the operation.
In conclusion, preoperative superselective renal artery embolization can be an effective tool to facilitate organ-preserving surgery in a case of a horseshoe kidney with renal cell carcinoma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download