INTRODUCTION
Bladder urothelial cell carcinoma is the most common malignancy among urothelial origin neoplasms. An accurate evaluation of the local extent of lesions (so called T-stage) is important to determine optimal therapeutic strategy and to predict treatment outcomes. Various image modalities can be used for the diagnosis and staging of urothelial cell carcinoma such as conventional cystoscopy, ultrasonography (US), computed tomography (CT) and magnetic resonance image (MRI). Routine two-dimensional (2D) gray scale US has been used as a screening modality of bladder disease due to its non-invasiveness, no radiation hazard, and easiness to apply. However, accuracies for T-staging of routine 2D US have been reported from 62% to 92%, showing lower accuracies to be associated with deeper tumors (1). Recent advances in image reconstruction and display technology have made three-dimensional (3D) volumetric US possible. This has provided additional information including a 3D impression of the pathological structure, unlimited opportunity to view in multiple planes and increased certainty of diagnosis with decreased subjectivity. It has also offered a more delicate anatomical delineation of small lesions which enables distinction of the superficial disease from the infiltrative disease (2).
3D volumetric US has become widely available in various ultrasound machines. In prior studies, 2- to 5-MHz convex or curvilinear transducers were used (345). Patients were asked to drink about 500 mL to 1000 mL of water and not to void for an hour before the US examination in order to perform it with the bladder fully distended (34). 2D US examinations were performed prior to 3D US to optimize the images and to adjust the region of interest. There are four main types of 3D US data acquisition systems: 1) tracked freehand systems, 2) untracked freehand systems, 3) mechanical assemblies, and 4) 2D arrays (6). The acquired 3D volume data sets display 3D images depending on rendering techniques such as surface rendering, multiplanar reformatting, and volume rendering techniques (6).
The purpose of this article is to present 3D volumetric US for bladder lesions and demonstrate various pathological conditions of the urinary bladder, ranging from bladder cancer to other lesions.
Superficial Polypoid Bladder Tumor
Superficial polypoid bladder tumor is defined as a tumor confined to the mucosa and lamina propria with a polypoid appearance, which embraces benign bladder lesions such as benign papillomas and bladder malignancy including superficial urothelial cancer (78).
On CT and MRI, superficial polypoid bladder tumor may appear as focal bladder wall thickening or an enhancing mass projecting toward the lumen without evidence of muscular or perivesical invasion (19). Current CT and MRI techniques cannot accurately resolve the various bladder wall layers, thereby accurate T staging of a superficial bladder tumor is limited (19).
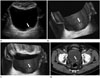
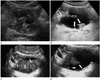
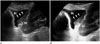
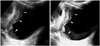
2D US can demonstrate intraluminal polypoid bladder lesion or focal bladder wall thickening. With the application of additional 3D volumetric US techniques, a small superficial bladder mass can be shown more clearly (Fig. 1). A previous study demonstrated that 3D volumetric US is a valuable method in a distinction between superficial bladder cancer (pTa) and muscle-invasive bladder cancer (pT1) (2). In the case of multiple bladder lesions, 3D volumetric US can distinguish each bladder nodule separately (Fig. 2).
There are limitations in detecting small and flat lesions or lesions located within the dome of the urinary bladder due to gas shadowing from interposed bowel loops, therefore conventional cystoscopy remains necessary (4). However, detection accuracies by US have known to range from 80% to 95% (10), and 3D volumetric US was more sensitive than 2D US in diagnosing bladder tumors in prior studies (311). Thus it could be predicted that this technique would play an important role in the detection of small lesions and could be performed when conventional cystoscopy is not suitable, such as in patients with severe urethral strictures (4).
Bladder Mass with an Indwelling Urinary Catheter
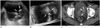
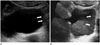
Patients with urinary tract neoplasm should maintain a urinary catheter due to a variety of reasons. However, a urinary catheter adjacent to a bladder mass may cause blurring of its boundary or the reverberation artifact, which results in decreased diagnostic performance of imaging modalities. In this case, 3D volumetric US can provide additional information of spatial structures and play a role in distinguishing a bladder lesion from the adjacent urinary catheter (Fig. 3).
Invasive Bladder Cancer
Invasive bladder cancer is defined as a malignant bladder tumor which invades muscularis propria and beyond (stage T2 or higher) (1912). Accurate staging of invasive bladder cancer is important for proper tumor management. Due to the fact that invasive bladder cancer is usually solid and pathologically poorly differentiated (13), it is not a good candidate for bladder preserving therapy (2).
CT and MRI are helpful in the diagnosis of invasive bladder cancer when perivesical fat invasion (T3) and direct invasion to adjacent organs, abdominal wall and pelvic wall (T4) are identified (9). However, in consideration of stage T2 tumors, current CT and MRIs have limitations in detecting and evaluating lesions due to poor resolution of the bladder's wall layers. On CT, retraction of the outer bladder wall at the site of a tumor may suggest deep muscle invasion (T2b); on MRI, T2a and T2b tumors can occasionally be differentiated using a combination of T2-weighted and contrast-enhanced T1-weighted sequences, because T2-weighted hypointensity of the bladder muscle is preserved in T2a tumors (1).
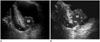
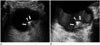
It is difficult to estimate the depth of invasion or to evaluate extravesical structures by US. US has also shown lower accuracies in T-staging in association with the deeper tumors (19), and current US still has a limitation in differentiating bladder wall layers (1). However, US is a real-time frontline examination without radiation hazards or invasiveness. Administration of 3D volumetric techniques provides a higher sensitivity when screening for bladder diseases and offers virtual sonographic cystoscopy with less invasiveness when compared with conventional cystoscopy (4). Sometimes, bladder cancer near the vesicoureteric orifices may cause ureteric obstruction presenting hydroureter or hydroureteronephrosis (1), which can be identified during a US exam (Fig. 4).
Thus, US with a 3D volumetric technique may play a role in evaluating the local extent of bladder lesions when considering stage T2 or less invasive bladder cancer, even if current US techniques have limitations in differentiating bladder wall layers and evaluation of extravesical structures (1).
Direct Invasion of Bladder by Malignancy from Adjacent Pelvic Organs
Direct bladder invasion by advanced cervical cancer, which is classified as FIGO stage IV and is not suitable for local treatment, is reported in about 11.5% of patients (14). An MRI has high accuracy in detection and quantification of bladder invasions, but it is expensive and not available in all medical institutions. A can US provide similar information about the evaluation of a bladder wall invasion when compared to that of an MRI (14). US features in each sequential stages of a bladder wall invasion is presumed to be a disruption of the endopelvic fascia without involvement of the inner bladder wall, thickened bladder wall and changes in bladder mucosa, or interruption of entire bladder wall (Fig. 5).
About 5% to 10% of colorectal cancers extend to the adjacent organ; urinary bladder is the most commonly involved (6). As described above, US features demonstrate sequential stages of invasion, ranging from disruption of mesorectal fascia to interruption of the entire bladder wall with mass formation (15).
Metastatic Cancer
Metastatic tumors of the urinary bladder from distant primary malignant foci are rare, and account for only 1.5% of all bladder tumors. The most common primary sites are gastric carcinoma, malignant melanoma, breast carcinoma, and lung carcinoma (16). Metastatic bladder tumors show either polypoid lesions similar to that of typical urothelial cell carcinoma, or focal wall thickening of the urinary bladder (Fig. 6) (16). As bladder metastasis is a late manifestation of malignancy, evidence of invasive primary neoplasm or other signs of a distant primary neoplasm is commonly identified on imaging studies (7).
Non-Tumorous Bladder Conditions
There are many non-tumorous bladder conditions mimicking bladder cancer due to the manifestation of focal bladder wall thickening (17), these include infections (cystitis, tuberculosis, acute schistosomiasis, malacoplakia, cystica glandularis), amyloidosis, endometriosis, and even benign bladder wall trabeculation. Flat bladder lesions can be easily missed on US but even when seen the appearances are often equivocal. In these cases, a sonographic differential diagnosis must be considered and further work up, including conventional cystoscopy, should be recommended.
Focal cystitis shows nonspecific bladder wall thickening and nodularity, therefore sometimes it is misdiagnosed as transitional cell carcinoma (Fig. 7).
A urinary stone at the ureterovesical junction (UVJ) with surrounding focal inflammatory changes due to irritation by the stone is shown as focal UVJ thickening, mimicking bladder cancer. In this case, a US examination will help to find any evidence of urinary stones, demonstrating echogenic structures with distal posterior acoustic shadowing (Fig. 8). Recent studies report that a transvaginal US in females is helpful in the detection of urinary stones (18).
CONCLUSION
Various diseases of urinary bladder (including bladder cancer, benign neoplasm, inflammatory condition, and even urinary tract stones) may be demonstrated as a polypoid, nodular bladder mass or as focal wall thickening of the urinary bladder; they are often easily confused with each other. In consideration of bladder cancer, a conventional cystoscopy is mandatory when attempting to determine a diagnosis. In addition a CT or MRI should be performed during an evaluation of extravesical structures because a US has limitations in the differentiation of bladder wall layers and the evaluation of a perivesical invasion.
Nevertheless, application of 3D volumetric US in this article has shown good depiction of bladder lesions with better spatial resolution. Especially when comparing other image modalities such as 2D gray scale US, CT, or MRI in cases of superficial and small lesions of the bladder. US is also well known to be a safe, widely available and less time-consuming imaging modality.
Application of 3D volumetric US provides additional information in the distinction and differentiation of various bladder diseases, including bladder cancer, with no harm to the patients.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download