Abstract
We report a case of recurrent sparganosis of the breast within 6 months following surgical removal of worms from the breast. The patient was referred to our hospital with a palpable mass in the right breast. On admission, breast ultrasonography revealed a tortuous tubular hypoechoic lesion with indistinct margins within a surrounding hyperechoic area, which strongly suggested sparganosis. We performed surgical excision and confirmed sparganosis. After 6 months, the patient detected a new mass in her right breast and visited our hospital. Breast ultrasonography revealed similar features in a different area of the same breast. We confirmed recurrent sparganosis surgically.
Sparganosis is an uncommon parasitic infection caused by the plerocercoid (second-stage) larvae of Spirometra erinacei (S. erinacei). The infection is transmitted by the ingestion of contaminated water or the consumption of raw or partially cooked fish, snake, or frog. Sparganosis is found worldwide, however, the majority of cases occur in East Asia, including Korea (1). Once humans become infected, the larvae can invade the muscle, intestine, eye, brain, and/or spinal cord. Patients may present with a subcutaneous mass or lump and vague pain (2). Recurrent sparganosis depends on the location of the worm and potential incomplete removal (3). Here, we report a case of recurrent sparganosis of the breast within 6 months following surgical removal of worms from the right breast.
A 62-year-old female patient visited our hospital with a chief complaint of a palpable right breast mass. She reported eating raw snake meat as a child. On admission, sonography was performed with a high-resolution (10-13 MHz) linear array transducer and an iU22 unit (Philips Ultrasound, Bothell, WA, USA). The scanning protocol included both transverse and longitudinal real-time imaging. Her breast ultrasonography revealed a tortuous tubular hypoechoic lesion with indistinct margins within a surrounding hyperechoic area, which strongly suggested sparganosis (Fig. 1). We surgically removed ivory-white, ribbon-like worms from the right breast and confirmed sparganosis. After 6 months, the patient detected a new mass in her right breast. Breast mammography revealed a lobulated, circumscrib-ed, isodense mass, and subsequent breast ultrasonography sh-owed similar features to those seen 6 months earlier, nevertheless in a different area of the same breast (Fig. 2). Once again, we surgically confirmed recurrent sparganosis. There was no evidence that the sparganum had invaded other tissues or organs such as extremities, intestine, brain, eyes, spine, pleura, or pericardium in this patient.
Breast sparganosis is a rare disease, representing less than 2% of all cases of sparganosis (4). The ultrasonographic findings of breast sparganosis may be useful for pre-operative diagnosis and patient management. The majority of breast sparganosis cases show characteristic features on ultrasonography such as multiple elongated, tubular, hypoechoic structures with or without internal heterogenic echogenicity. Hyperechoic, perilesional fat is presumably produced by chronic inflammatory reactions. Color Doppler examination typically does not show vascular flow within the mass, but may evidence increased vascularity in patients with pain (5). In spite of characteristic sonographic findings, breast sparganosis can mimic malignancy, especially in patients with previous or current malignant disease (6). Complete surgical removal is the treatment of choice and provides a definite diagnosis.
A previous report hypothesized that recurrent sparganosis depends on the location of the worms and potential incomplete removal (3). In the case of recurrent lower extremity and breast sparganosis, authors suggested the possibility of incomplete surgical removal of sparganosis in the upper medial portion of the right breast 2 years earlier, resulting in subsequent migration towards the left breast, and then distantly to the lower extremites (7). Oh et al. (8), reported a case of pulmonary sparganosis in a patient with previous surgical excision of recurrent muscular and subcutaneous spargnosis. If two or more spargana exist in a patient, complete surgical resection may be difficult, leaving room for recurrence of sparganosis by remnant worms after the surgery. Recurrence may also be possible if the scolex part was cut in a previous surgery, retained, and regenerated thereafter (9). The longevity of S. erinacei is thought to be less than 1 year, however, some articles have reported finding a live worm after more than 10 years (10). Thus, any patient diagnosed with breast sparganosis should be followed for a certain period of time, since recurrence is possible, as seen in our case. Moreover, considering that sparganum larvae can penetrate anywhere in the body, the possibility of simultaneous involvement of other tissues, such as the abdominal wall and extremities, should be considered.
Figures and Tables
Fig. 1
A 62-year-old woman with breast sparganosis.
A, B. Grayscale coronal and sagittal ultrasonography reveals a tortuous tubular hypoechoic lesion with indistinct margins within a surrounding hyperechoic area in the right breast at the 9 o'clock position.
C. Color Doppler ultrasonography shows increased peripheral vascularity of the lesion.
D. Pathological examination demonstrates ivory-white, ribbon-like worms from the right breast, confirming the diagnosis of sparganosis.
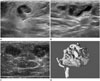
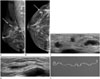
Fig. 2
The same patient with recurrent breast sparganosis 6 months following surgery.
A, B. Breast mammography reveals a lobulated, circumscribed, isodense mass in the right breast upper outer quadrant (arrow).
C, D. Grayscale ultrasonography shows a tortuous tubular hypoechoic region in a different area of the right breast (at the 5 o'clock position).
E. Pathological examination demonstrates ivory-white worms from the right breast, confirming the diagnosis of sparganosis.
References
1. Cho SY, Bae JH, Seo BS. Some aspects of human sparganosis in Korea. Korean J Parasitol. 1975; 13:60–77.
2. Yoon HS, Jeon BJ, Park BY. Multiple sparganosis in an immunosuppressed patient. Arch Plast Surg. 2013; 40:479–481.
3. Koo M, Kim JH, Kim JS, Lee JE, Nam SJ, Yang JH. Cases and literature review of breast sparganosis. World J Surg. 2011; 35:573–579.
4. Moon HG, Jung EJ, Park ST. Breast sparganosis presenting as a breast mass with vague migrating pain. J Am Coll Surg. 2008; 207:292.
5. Park HJ, Park NH, Lee EJ, Park CS, Lee SM, Park SI. Ultrasonographic findings of subcutaneous and muscular sparganosis. J Korean Soc Radiol. 2009; 61:183–187.
6. Kim JW, Hwang MS. Sparganosis of the breast that mimicked metastasis: a case report. J Korean Soc Ultrasound Med. 2011; 30:59–62.
7. Choi SJ, Park SH, Kim MJ, Jung M, Ko BH. Sparganosis of the breast and lower extremities: sonographic appearance. J Clin Ultrasound. 2014; 42:436–438.
8. Oh YJ, Kim MJ, Cho JH, Cha CW, Kim DH, Oh MJ, et al. A case of pulmonary sparganosis in a patient with a history of recurrent sparganum infections. Tuberc Respir Dis. 2009; 67:229–233.
9. Lee YI, Seo M, Park HW. Recurred sparganosis 1 year after surgical removal of a sparganum in a Korean woman. Korean J Parasitol. 2014; 52:75–78.
10. Kubota T, Itoh M. Sparganosis associated with orbital myositis. Jpn J Ophthalmol. 2007; 51:311–312.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download