Abstract
Desmoplastic small round cell tumor (DSRCT) is a highly aggressive malignant small cell neoplasm occurring mainly in the abdominal cavity, but it is extremely rare in the pleura. In this case, a 15-year-old male presented with a 1-month history of left chest pain. Chest radiographs revealed pleural thickening in the left hemithorax and chest computed tomography showed multifocal pleural thickening with enhancement in both hemithoraces. A needle biopsy of the left pleural lesion was performed and the final diagnosis was DSRCT of the pleura. We report this unusual case arising from the pleura bilaterally. The pleural involvement of this tumor supports the hypothesis that it typically occurs in mesothelial-lined surfaces.
Desmoplastic small round cell tumor (DSRCT) is a highly aggressive malignant small cell neoplasm that tends to affect adolescents and young adults and occurs predominantly in the abdominal cavity, including the pelvis and omentum (1, 2). Other primary sites are rare and have included the paratesticular region, pleura, posterior cranial fossa, soft tissues, bone, ovary, and kidney (2, 3, 4). DSRCT in the lung is extremely rare. This distinct clinicopathological entity was first described by Gerald and Rosai (1) in 1989.
A DSRCT is composed of small round tumor cells of uncertain histogenesis, associated with prominent stromal desmoplasia and polyphenotypic differentiation. This article reports bilateral tumors in the pleura of a 15-year-old male.
A 15-year-old male presented with a 1-month history of sharp pain in the left lower chest, which occasionally woke him. He was a nonsmoker, as were his parents. He had no serious medical or surgical history and he had grown up in an ordinary residential and social environment. He had no shortness of breath or cough. There was no history of weight loss, fever, or night sweats.
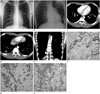
Chest radiographs showed a soft-tissue mass in the left mid hemithorax (Fig. 1A). The mass was at an obtuse angle to the chest wall. The long diameter of the mass was approximately 9 cm. There was no other abnormality in the lung parenchyma or bony thorax. In the left down decubitus view, the mass did not shift position or change contour (Fig. 1B).
Chest computed tomography (CT) revealed multifocal nodular pleural thickening in the lateral, posterior aspect of the left hemithorax and posterior aspect of the right lower hemithorax (Fig. 1C-E). In the pre-contrast image, the pleural lesion showed homogeneous soft tissue attenuation and there was no calcification or pleural effusion. After contrast enhancement, the thickened pleura generally showed homogeneous enhancement, with attenuation similar to that of the back muscles and a subtle low density was seen in the lesion in the lateral left hemithorax. The mediastinal and hilar lymph nodes were not enlarged. In the scanned portion of the abdomen, there was no mass, lymphadenopathy, or ascites. From these imaging findings, we suspected fibromatosis of the pleura and a localized fibrous tumor because it appeared as a well-defined relatively homogeneous soft tissue attenuated mass in the pleura. We also considered a primitive neuroectodermal tumor (PNET) because there was no calcification and it contained subtle low-density foci.
About 2 weeks later, a needle biopsy of the largest pleural lesion in the left hemithorax was performed and we obtained two pieces of grayish elongated soft tissue, with the larger measuring 1.9 cm in length. Microscopic examination showed nests or clumps of small cells set in abundant desmoplastic stroma. The tumor cells had small hyperchromatic nuclei with little cytoplasm and indistinct nucleoli. Mitotic figures were common (Fig. 1F). Immunohistochemically, the tumor cells were positive for CD56, desmin, synaptophysin, and vimentin, and negative for cytokeratin, epithelial membrane antigen, neuron specific enolase, and thyroid transcription factor-1 (Fig. 1G, H).
The patient refused surgery and was treated with vincristine, doxorubicin, and cyclophosphamide, alternating with ifosfamide and etoposide. The patient died from sepsis 16 months after the initial diagnosis.
A DSRCT is a rare, aggressive malignancy, typically occurring in young adults and more common in males. It is most commonly reported in children and young adults between 15 and 35 years of age, with a male-to-female ratio of 4:1. This tumor has poor median survival rates (5).
The majority of cases occur in the abdominal serosa, including the omentum and paravesical space and this is known as an intra-abdominal desmoplastic tumor (2). There have been a few cases reported in the pleural cavity, paratesticular region, and even intracranially (2, 3, 4). The clinical signs are non-specific and vary according to the involved organ. In most cases, DSRCT presents as an abdominal mass with peritoneal seeding. A frequently associated symptom is crampy abdominal pain (5). With a pleural presentation, common associated symptoms are chest pain and dyspnea (3).
Computed tomography and magnetic resonance imaging (MRI) are relatively useful tools for evaluating DSRCT, although the DSRCT image findings are nonspecific. The most common CT finding of pleural DSRCT is single or multiple soft-tissue pleural masses with combined pleural effusion. Pleural nodularity is another common finding. The low-density area often represents necrosis, hemorrhage, or fibrous components. Calcification can be seen in both primary and metastatic tumors. Due to the various fibrotic, necrotic, and calcified components, DSRCT shows inhomogeneous attenuation in pre-contrast CT. Contrast enhancement is relatively weak, probably due to the fibrous component caused by the desmoplastic reaction. MRI is useful for assessing tumor extent. On MRI, DSRCT often shows a heterogeneous high signal intensity on T2-weighted images and iso to low signal intensity on T1-weighted images. After administering gadolinium, it presents as a heterogeneously enhancing mass. On positron emission tomography, DSRCT shows increased fluorodeoxyglucose uptake (6).
The diagnosis of pleural DSRCT can be established from the clinical, histological, and immunohistochemical features. The histogenesis of DSRCT is uncertain, but its predilection for serosal involvement suggests a mesothelial origin.
A typical feature of DSRCT is the angulated nests of small round cells embedded in a cellular fibroblastic stroma. Grossly, areas of central low attenuation on CT might correspond to hemorrhage or necrosis. Immunohistochemistry is helpful for diagnosing DSRCT. Immunohistochemically, the co-expression of cytokeratins, vimentin and desmin is a characteristic feature. The perinuclear dot-like staining for vimentin is unique.
The differential diagnosis of pleural DSRCT includes malignant lymphoma, classical neuroblastoma, rhabdomyosarcoma, rhabdoid tumor, PNET, and malignant mesothelioma. The gross pattern and radiological findings of DSRCT mostly resembled malignant mesothelioma. However, primary mesothelioma is extremely rare in children and young adults. Radiologically, malignant mesothelioma is also often combined with a massive pleural effusion, but it can have a circumferential pattern of involvement, with disease extending along the fissural, mediastinal, or pericardial pleura. In our case, there was no pleural effusion or circumferential pattern of involvement. In addition, the patient was younger than commonly seen in mesothelioma. Histologically, the dot-like immunostaining for vimentin and negative for CK5/6 and calretinin distinguish DSRCT from the small cell variant of mesothelioma (3).
Primitive neuroectodermal tumors are also similar to DSRCT, in the age of presentation and the imaging, histological, and cytological findings. Radiologically, the findings of PNET are similar to those of DSRCT from the perspective of an extraparenchymal soft-tissue-density mass with a pleural effusion, often including a necrotic portion. However, in PNET, calcification is rare and rib destruction is often combined (7). In our case, the mass contained a low-density portion and there was no calcification or pleural effusion. However, no rib destruction, which is common with PNET, was seen. Histologically, it was composed of small round, undifferentiated blue cells with scant cytoplasm. Immunohistologically, PNET usually show unidirectional differentiation toward neural elements, while DSRCT shows multidirectional differentiation toward muscle, neural, and epithelial elements.
Malignant non-Hodgkin's lymphoma is also similar to DSRCT. On imaging, pleural nodules with focal or diffuse pleural thickening with homogeneous enhancement accompanied by pleural effusion can be seen (8). However, it often has associated mediastinal and hilar lymphadenopathy, unlike DSRCT (8). In our case, there was no lymphadenopathy and the image findings resembled DSRCT more than malignant non-Hodgkin's lymphoma.
Most neuroblastomas occur before 5 years of age, and characteristically present with invasion through the neural foramina, giving a dumbbell appearance due to their origin from sympathetic nervous tissue (7). In our case, the patient was older than most neuroblastoma patients and the tumor did not have a dumbbell shape.
The prognosis of DSRCT remains poor and the 5-year survival rate is less than 15% (5). There is no recommended treatment for DSRCT. Aggressive anticancer treatment is thought to contribute to relatively long-term survival (4). Aggressive surgery can be used to reduce the tumor size before or after chemotherapy and complete surgical excision seems to improve survival (2). Radiation therapy can be used to treat DSRCT (2). Despite the poor overall prognosis, there are some cases of long-term survival and they common involve early aggressive anticancer treatment (4). There are some new approaches to treating DSRCT, such as molecular targeted therapy and immunotherapy (9, 10).
In summary, DSRCT is a rare, highly aggressive malignancy that typically involves the abdominal cavity, but is rare in the pleura. The imaging findings of DSRCT in the pleura are nonspecific and it is difficult to suspect DSRCT. Various treatments have been attempted for DSRCT, but the prognosis is quite poor despite aggressive combination therapy. Nevertheless, there are some reports of long-term survival and they are common with initial aggressive anticancer treatment (4). Therefore, the early detection of DSRCT is very important for achieving long-term survival. Consequently, DSRCT could be considered in the differential diagnosis of multifocal pleural thickening containing necrosis or calcification with inhomogeneous enhancement and an accompanying pleural effusion, especially in adolescent males.
Figures and Tables
Fig. 1
Chest radiographs (A, B), chest CT (C-E), and microscopic features (F-H) of desmoplastic small round cell tumor in a 15-year-old male.
A, B. Chest radiograph posteroanterior (A) and left lateral decubitus (B) views show a soft tissue mass (arrow) at the left mid hemithorax.
C, D. Transverse CT image of the chest shows multifocal pleural plaque with enhancement in both hemithoraces (left hemithorax, arrow; right hemithorax, arrowhead).
E. Coronal reformatted CT image shows a pleural plaque at the lateral aspect of the left hemithorax.
F. Photomicrograph of hematoxylin and eosin-stained specimen (× 100) shows cellular small round cell nests within dense fibrous stroma.
G, H. Positive immunoreactivity for CD56 (× 200) (G) and vimentin (× 200) (H) is seen on the tumor cell membranes.
References
1. Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989; 9:177–118.
2. Biswas G, Laskar S, Banavali SD, Gujral S, Kurkure PA, Muckaden M, et al. Desmoplastic small round cell tumor: extra abdominal and abdominal presentations and the results of treatment. Indian J Cancer. 2005; 42:78–84.
3. Parkash V, Gerald WL, Parma A, Miettinen M, Rosai J. Desmoplastic small round cell tumor of the pleura. Am J Surg Pathol. 1995; 19:659–665.
4. Ostoros G, Orosz Z, Kovács G, Soltész I. Desmoplastic small round cell tumour of the pleura: a case report with unusual follow-up. Lung Cancer. 2002; 36:333–336.
5. Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg. 2005; 40:251–255.
6. Kis B, O'Regan KN, Agoston A, Javery O, Jagannathan J, Ramaiya NH. Imaging of desmoplastic small round cell tumour in adults. Br J Radiol. 2012; 85:187–192.
7. Xu Q, Xu K, Yang C, Zhang X, Meng Y, Quan Q. Askin tumor: four case reports and a review of the literature. Cancer Imaging. 2011; 11:184–188.
8. Dynes MC, White EM, Fry WA, Ghahremani GG. Imaging manifestations of pleural tumors. Radiographics. 1992; 12:1191–1201.
9. Li H, Smolen GA, Beers LF, Xia L, Gerald W, Wang J, et al. Adenosine transporter ENT4 is a direct target of EWS/WT1 translocation product and is highly expressed in desmoplastic small round cell tumor. PLoS One. 2008; 3:e2353.
10. Modak S, Gerald W, Cheung NK. Disialoganglioside GD2 and a novel tumor antigen: potential targets for immunotherapy of desmoplastic small round cell tumor. Med Pediatr Oncol. 2002; 39:547–551.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download