Infection with Mycobacterium tuberculosis has a wide array of manifestations. Although the involvement of liver, spleen, bowel, and mesenteric lymph nodes is commonly seen in miliary tuberculosis, tuberculosis affecting only the peripancreatic lymph nodes is a rare clinical entity (1). Correct diagnosis is crucial in establishing adequate management and treatment. Its occurrence may pose a diagnostic challenge due to its similarity with a pancreatic neoplasm. We present a rare case of peripancreatic tuberculous lymphadenopathy which was misdiagnosed as a pancreatic neoplasm.
Case Report
A 40-year-old man presented at our institution with an incidental pancreatic mass revealed by abdominal sonography as part of a regular health checkup. He had no symptoms or past history including pulmonary tuberculosis and similarly, showed no weight loss, fever, chills, nausea, or vomiting. Further, he had no family history of malignancy or inherited disease. His physical examination was unremarkable and all laboratory tests were also normal including no serological indication of active HIV infection.
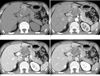
Pancreatobiliary CT imaging was performed, which revealed a well-defined, lobulating contoured heterogeneous mass, located between the head of pancreas, the lesser curvature of the gastric body, and the left lobe of the liver (Fig. 1). The size of the mass was approximately, 6×4.4 cm, and more hypoattenuated than pancreatic parenchyma in all phases of a dynamic-enhanced CT scan. In a pre contrast scan, a thin linear and punctuate calcifications were noted at the periphery of the mass. Amorphous high attenuated areas were also presented in the mass, which measured approximately 70-80 HU with no enhancement. Hence, we presumed that high attenuated areas were hemorrhage or calcified component. In contrast, an enhancement scan found that internal low density lesions showed no enhancement, but peripheral wall and internal septa showed delayed enhancement. An indentation was found in the pancreatic parenchyma, which was adjacent to the mass, whereas no detectable fat plane was found between the mass and the pancreas and the pancreatic duct was not dilatated. Small calcifications were found in the retropancreatic region and the left small bowel mesentery.
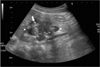
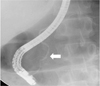
Additional findings were rendered as follows: complementary abdominal sonography showed the internal architecture of the mass, with calcifications, septa, and an anechoic cystic portion (Fig. 2). Endoscopic retrograde cholangiopancreatography (ERCP) showed grossly normal papilla, and no remarkable findings on both the pancreatic and common bile ducts (Fig. 3).
Our initial diagnosis of the results was a cystic pancreatic neoplasm, which was a solid-pseudopapillary tumor or mucinous cystic neoplasm of the pancreas, which may present as a peripheral calcification and internal hemorrhage or mucinous component.
A celiac and superior mesenteric artery angiography was performed to determine the operability of the pancreatic mass, as well as the common and proper hepatic artery. The main portal vein showed displacement without any evidence of angioinvasion (Fig. 4).
Since we did not have a firm preoperative diagnosis, the patient underwent an exploratory laparotomy. Intraoperative exploration revealed a smooth, fleshy, solid mass measuring 10 cm, located in the lesser sac portion of the suprapancreatic area, which was focal firm adherent to the superior border of the. No remarkable finding was found in the entire pancreas. The mass was totally excised, along with some adjacent some lymph nodes. Yellow cheese-like pus was drained from the mass during the procedure.
Histopathologic examination of the mass revealed lymphoid tissue; the inner surface of the cyst wall was filled with thick yellowish dirty necrotic fluid. The cystic wall was thickened by up to 0.3 cm.
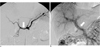
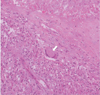
The final histopathologic diagnosis was chronic granulomatous lymphadenitis with caseation necrosis and central cystic change, consistent with tuberculosis (Fig. 5). A subsequent TB-PCR showed a positive reaction for mycobacterium tuberculosis.
After diagnosis of the peripancreatic tuberculosis, the patient began a tuberculosis medication regime (INH 300, RFP 600, EMB 1200, PZA 1500) and was discharge from our institution without any postoperative complication.
Discussion
Tuberculosis is a re-emerging global emergency which is further complicated by AIDS/HIV infection and the use of immunosuppressant drugs (2). In particular, abdominal tuberculosis is a diagnostic challenge to physicians because it mimics a broad spectrum of miscellaneous diseases (23).
The most common manifestation of abdominal tuberculosis is lymphadenopathy, which is usually associated with gastrointestinal tuberculosis and less commonly with peritoneal or solid organ involvement. Lymphadenopathy can occur as an isolated manifestation without other evidence of abdominal involvement (4), or can also be the only sign of the disease, especially in the periportal region (56). Kim et al. reported that the peripancreatic area was the most commonly affected site, followed by the periportal lymph nodes (4).
Commonly involved mesenteric root, celiac, porta hepatis, peripancreatic lymph nodes is explained by the drainage of the ingested infected material by the lymphatics of the ileocecal region, jejunum, ileum and right side of the colon (5). Retroperitoneal lymph nodes are relatively spared and their involvement rarely occurs in isolation (2). In the absence of any detectable lesion in the lungs, liver, bones, and any other abdominal organs, the pathogenesis of peripancreatic lymph node involvement is difficult to explain. It is widely speculated that tubercle bacilli reaches the peripancreatic lymph node as a result of lymphohematogeneous dissemination from an occult focus in the lung (37).
Tuberculous lymphadenopathy patterns vary widely (5); on US, the lymph nodes are either discrete or appear as matted conglomerated masses. Enlarged nodes usually contain central hypoechic areas (56). Moreover, lymph node enlargement is better assessed on CT than on US (456). Caseation and liquefaction substances at the center of the enlarged lymph node have low attenuation; as opposed to peripheral inflammatory lymphatic tissue, which has higher attenuation on enhanced CT (5). The enhancement pattern of tuberculous lymphadenopathy was reported as three or four different patterns after contrast administration (6). Peripheral enhancement, the most frequent pattern, is characterized by peripheral enhancement, with or without low attenuation spots at the center of the nodes; the other include heterogeneous enhancement due to the presence of focal non enhanced intranodal areas, homogeneous enhancement, and non enhanced-low attenuation nodes seen only in patients with AIDS (56).
Solitary or isolated tuberculous peripancreatic lymph node involvement is very rare. It could be attributed to one of four distinct clinical scenarios: the infection may (1) produce pancreatitis, (2) cause obstructive jaundice, (3) lead to gastrointestinal bleeding, or as in the present case, (4) and mimic a pancreatic neoplasm as a discrete mass (8).
If peripancreatic tuberculous lymphadenopathy is close to the pancreas and presents as complex cystic lesions, just as in our case, and doesn't make distinguishing it from pancreatic cystic neoplasm any easier (9). Because the differential diagnosis of a cystic pancreatic mass is broad and includes a serous cystic adenoma, mucinous cystic neoplasm, solid and pseudopapillary tumor, intraductal papillary mucinous neoplasm, and pancreatic pseudocyst, amongst others, CT scans are often unreliable for diagnosis. Unfortunately, to date, no studies have demonstrated a reliable imaging technique to accurately distinguish tuberculosis from other pancreatic pathology (8).
In that situation, MRI could be helpful for the differential diagnosis of peripancreatic tuberculosis and pancreatic neoplasm (4). Lesions initially diagnosed as pancreatic cystic tumor on abdominal CT could be well differentiated from displaced normal pancreatic parenchyma by the difference in signal intensity and enhancement pattern between the abutting lesions.
In addition, at an early stage of formation of tuberculoma, the mass showed isointensity on T1- and T2 weighted images due to abundant inflammatory cells and a capsule poor in collagen. At a later stage, the lesion shows low signal intensity on T2-weighted images due to fibrosis, scar tissue, and free radicals, and peripheral ring or nodular contrast enhancement. Noninvasiveness to adjacent structures may be another unique feature of the disease (4). Despite the considerable bulk of adenopathy in some patients, urinary and gastrointestinal obstruction has not been reported. However, biliary obstruction may present secondary to direct ductal compression by infected nodes in association with periductal inflammation and stricture (6), or external compression (10).
Peripancreatic tuberculosis is rare and could present a diagnostic challenge. In endemic countries, tuberculosis should be considered and aimed for in making a definite diagnosis for a tumoral cystic mass with calcification located in the peripancreatic region, especially in young patients.
However, if physicians were aware of these rare clinical entities and some helpful radiologic findings, appropriate investigations could be performed with minimal invasiveness such as image-guided FNA or laparoscopic biopsy. Diagnosis of pancreatic tuberculosis without performing a laparotomy is possible and the disease can be more effectively treated with antituberculous drugs.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download