Abstract
Sparganosis of the breast is a quite rare parasitic infection of humans and presents as soft tissue masses that mimic breast malignancy or benign tumor, such as fibroadenoma. We present here a case of histologically confirmed breast sparganosis in the upper inner quadrant of the right breast with coexisting breast cancer in the ipsilateral breast upper outer quadrant. Ultrasonography of breast sparganosis showed a well defined, tubular hypoechoic mass with discrete multilayered wall and tubule-in tubule appearance, surrounded by heterogenous hyperechoic areas in the subcutaneous fat layer of the breast. MRI revealed an elongated tubular structure with persistent and progressive enhancement. This is the second report concerned with the MRI and ultrasonographic findings of breast sparganosis and the first report of breast sparganosis in a patient with ipsilateral breast cancer.
Sparganosis is a rare parasitic infection of humans caused by the larval form of the pseudophyllidean tapeworm Spirometra (1). Humans are usually aberrant hosts (2). The route of infection is ingestion of raw meat of snakes or frogs, or drinking contaminated water (2). The clinical finding of sparganosis is the presence of subcutaneous masses in the abdominal wall, scrotum, lower extremities, chest wall, brain and breast (3). There are few case reports in the English literature on the breast sparganosis (3). We report here on the MRI and ultrasonographic findings of histologically confirmed breast sparganosis in a patient with coexisting ipsilateral breast cancer. This is the second report about MRI and ultrasonographic findings on the breast sparganosis (4) and the first report of breast sparganosis with coexisting ipsilateral breast cancer.
A 56-year-old woman who had palpable masses in the upper inner quadrant and the upper outer quadrant of the right breast with 8 months duration, visitied a local clinic and was suspected of having soft tissue tumors. She was then transferred to our hospital. The patient had no prior history of breast trauma and no family history of breast cancer. In addition, she had no history of eating either frogs or snakes. However she had a history of ingesting raw meat and drinking water from a spring when climbing mountains. The clinical breast examination revealed hard, non-tender masses in the 2 o'clock position of the upper inner quadrant and the 10 o'clock position of the upper outer quadrant of the right breast. There was no evidence of axillary or supraclavicular adenopathy. The results of laboratory tests, including complete blood count, liver function test, urinalysis, and tumor marker studies, were within the normal reference ranges.
Mammographic and sonographic evaluations were performed. Mammography revealed scattered fibroglandular densities and a focal asymmetry with a well defined cord-like isodense structure without microcalcification in the right breast upper inner quadrant (Fig. 1). Incidental benign punctate calcifications were noted in the right breast upper outer quadrant (Fig. 1). Ultrasound images showed a 9 mm sized oval shaped, indistinct margined, hypoechoic mass in the 10 o'clock position of the right breast upper outer quadrant (Figs. 2A, B). This lesion was not apparent on the images of the mammography. Based on the US imaging findings, the lesion was thought to be of low suspicious abnormality and was classified as Breast Imaging Reporting and Data System (BI-RADS) category 4a. Ultrasonography demonstrated another well defined tubular hypoechoic mass with discrete multilayered wall and tubule-in tubule appearance, surrounded by heterogenous hyperechoic areas of the subcutaneous fat layer in the 2 o'clock position of the ipsilateral right breast upper inner quadrant (Figs. 2C, D). Color Doppler examination showed no vascular flow within the mass. The lesion of the right breast upper inner quadrant seemed to be a probably benign finding but primary malignancy or metastatic lesion could not be excluded.
For evaluation of the two masses of the right breast, MRI was performed using a 1.5T MRI System (Magnetom Symphony; Siemens, Erlangen, Germany). Axial fat-saturated fast spin-echo T2 weighted image (TR/TE 5800/59), dynamic axial fat-saturated T1 weighted images (TR/TE 5.2/2.4) with gadolinium DTPA enhancement (Magnevist, Schering, Berlin, Germany) at every 1 minute for 6 minutes and postcontrast T1 weighted maximum intensity projection (MIP) images (TR/TE 5.2/2.4) were acquired.
Contrast enhanced T1 weighted images showed a 9 mm sized oval shaped, irregular margined mass with homogeneous enhancement in the right breast upper outer quadrant (Fig. 3A). Postcontrast T1 weighted maximum intensity projection (MIP) images showed prominent abnormal focus of enhancement of the lesion (Fig. 3B). Enhancement curve for the ROI of the selected region of the mass demonstrated a type III rapid rise and washout pattern, involving an initial increase and subsequent decrease in signal intensity (Figs. 3C, D). Another elongated tubular structure and cross-sectional round or oval lesion was seen in the ipsilateral right breast upper inner quadrant (Fig. 3E). It showed persistent and progressive enhancement on dynamic contrast enhanced T1 weighted images (Fig. 3B). Enhancement curve for the ROI of the mass showed a type I persistent pattern with a continuous increase in signal intensity (Figs. 3F, G).
A 14-gauge core biopsy was performed for two masses with ultrasound guidance. Histopathologically, the lesion in the right breast upper outer quadrant was confirmed as invasive ductal carcinoma. In the ipsilateral right breast upper inner quadrant, the presence of sparganum organism with chronic granulomatous inflammation was revealed on the microscopic findings.
The patient underwent excision of all the masses. An ivory opaque cord-like tapeworm with active movement was extracted in the right breast upper inner quadrant. It measured 24.0 cm × 0.7 cm in size, and the presence of scolex was confirmed in this segment (Fig. 4A). The worm was identified as a sparganum of Spirometra sp. The microscopic evaluation revealed a sparganum organism with surrounding acute and chronic granulomatous inflammation (Fig. 4B) and it was consistent with a diagnosis of breast sparganosis. The final pathologic diagnosis was a breast sparganosis in the upper inner quadrant of the right breast. Coexisting invasive ductal carcinoma was confirmed in the upper outer quadrant of the ipsilateral breast.
Spargana can migrate to any part of the human body including the subcutaneous tissue of the abdominal wall, chest wall, urogenital organs, extremities, central nervous system, chest, orbital region, rarely brain and oral cavity (5). Breast sparganosis is extremely rare. The most common route of human infection is ingestion of raw meat of snakes or frogs, or drinking of contaminated water (2). Our patient occasionally ingested raw meat and drank water from a spring.
Breast sparganosis presents as soft tissue masses, as in the present case, and may therefore be confused with neoplastic masses (6). Its mammographic features are usually lobular, solid mass without microcalcifications, which are similar to the features of circumscribed breast cancer or benign tumor, such as fibroadenoma (17). Therefore, they constitute a diagnostic dilemma, particularly in the breast cancer patient, like our case. According to the reports on breast sparganosis by Park et al. (3), Kim et al. (4), Moon et al. (8), breast sparganosis may present as breast masses requiring a biopsy to exclude malignancy. In our case, core biopsy was performed on the mass infected by sparganosis and the malignant mass, because neoplastic disease could not be excluded. However, in patients with a breast mass with mammographic and ultrasonographic features of breast sparganosis, direct surgical excision should be considered rather than percutaneous needle biopsy (8). And a confirmatory diagnosis of breast sparganosis should be established by extracting the worm or by examining surgical pathology specimens (3).
According to the previously published reports, the ultrasonographic findings of breast sparganosis were similar to those of musculoskeletal sparganosis, where linear echogenicity with a 'dot and dash' pattern was seen in some portions of the tract (25). Our case demonstrated a well defined, tubular hypoechoic mass with discrete multilayered wall and tubule-in tubule appearance, presumably caused by the presence of the worm. There was no vascular flow within the mass. The surrounding poorly defined heterogenous increased echogenicity of the subcutaneous fat layer of the breast was presumably produced by chronic inflammatory reactions. Our US findings are similar to those of the report of breast sparganosis by Kim et al. (1), Park et al. (3) and Kim et al. (9). According to Kim et al. (1), a hypoechoic tubular structure is due to the echogenicity of the worm itself and the increased echogenicity of the surrounding structure is due to combined chronic granulomatous inflammation, a finding in accord with our case.
To the best of our knowledge, only one case on the MRI findings of breast sparganosis has been previously reported (4). Our morphologic MRI findings of elongated tubular structures and cross-sectional round or oval lesion were consistent with the findings reported by Kim et al. (4). However, the contrast enhancement pattern of the lesion can be variable. In contrast to persistent and progressive enhancement of the lesion in our case, Kim et al. reported delayed subtle enhancement of the lesion on MR images (4). Breast sparganosis may show variable enhancement due to the variable extent of the surrounding inflammatory reaction and perilesional edema, as similar to musculoskeletal sparganosis (210). In our case, breast sparganosis showed a type I persistent enhancement curve for the ROI. The type I pattern e nhancement curve for the ROI, is usually associated with a benign finding (83% benign, 9% malignant) with sensitivity and specificity of 52.2% and 71% for indication of a benign lesion, respectively (10). However, a multi-institutional trial by Schnall et al. reported that 45% of the lesions with persistent enhancement kinetics were proved to be cancers (10). Therefore, exclusion of cancer on the basis of persistent enhancement (a type I curve) alone is not recommended on the interpretation and would lead to false-negative results, because of the overlap in enhancement characteristics between benign and malignant lesions (10).
Findings of elongated tubular structures may also be obtained in other types of diseases, such as, ectatic ducts, radiation edema, superficial thrombophlebitis, and congestive heart failure (17). We believe that the unique wall shaped tubular structures with granulomatous inflammation may be helpful in differentiation of breast sparganosis from other diseases.
In conclusion, this case suggests that the US and MR morphologic findings are helpful to diagnose breast sparganosis and to differentiate it from breast malignancy or benign tumors, particularly in a patient with coexisting breast cancer, when an elongated tubular and cross sectional oval lesion that shows a multilayered wall with tubule-in tubule appearance is noted. Knowledge of this rare entity may be helpful in the diagnosis and clinical management of breast sparganosis.
Figures and Tables
Fig. 1
A 56-year-old woman with two palpable masses in the right breast.
Cranio-caudal (A) and medio-lateral oblique (B) mammographic views of the right breast show a focal asymmetry with a well defined cord-like isodense structure (white arrows) without microcalcification in the right breast upper inner quadrant. The black arrow indicates incidental benign punctate calcifications in the right breast upper outer quadrant.
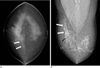
Fig. 2
A 56-year-old woman with coexisting invasive ductal carcinoma (A, B) and breast sparganosis (C, D) in the right breast.
A, B. Ultrasound images show a 9 mm sized oval shaped, indistinct margined, hypoechoic mass (arrowhead) in the 10 o'clock position of the right breast upper outer quadrant. This lesion was not apparent on the images of the mammography. Based on the US imaging findings, it is thought to be classified as Breast Imaging Reporting and Data System (BI-RADS) category 4a.
C, D. Ultrasound images show a well defined tubular hypoechoic mass with discrete multilayered wall and tubule-in tubule appearance (asterisk), surrounded by heterogenous hyperechoic areas of the subcutaneous fat layer (arrows) in the 2 o'clock position of the ipsilateral right breast upper inner quadrant.
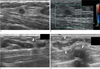
Fig. 3
A 56-year-old woman with coexisting invasive ductal carcinoma (A-D) and breast sparganosis (EH) in the right breast.
A. Dynamic axial fat saturated T1 weighted images (TR/TE 5.2/2.4) with gadolinium DTPA enhancement show a 9 mm sized oval shaped, irregular margined mass with homogeneous enhancement (open arrowhead) in the right breast upper outer quadrant.
B. Postcontrast T1 weighted maximum intensity projection (MIP) image (TR/TE 5.2/2.4) shows prominent abnormal focus of enhancement (open arrowhead) in the right breast upper outer quadrant.
C, D. Enhancement curve for the ROI of the selected region of the mass shows a type III rapid rise and washout pattern, involving an initial increase and subsequent decrease in signal intensity. The vertical axis indicates the percentage of enhancement, and the horizontal axis indicates the time in seconds.
E. Axial fat-saturated fast spin-echo T2 weighted image (TR/TE 5800/59) shows an elongated tubular structure (arrowheads) with cross-sectional round or oval appearance in the ipsilateral right breast upper inner quadrant.
F. Dynamic axial fat saturated T1 weighted images (TR/TE 5.2/2.4) with gadolinium DTPA enhancement at every 1 minute for 6 minutes show persistent and progressive enhancement of the mass (arrowheads) in the right breast upper inner quadrant.
G, H. Enhancement curve for the ROI of the mass shows a type I persistent enhancement pattern with a continuous increase in signal intensity. The vertical axis indicates the percentage of enhancement, and the horizontal axis indicates the time in seconds.
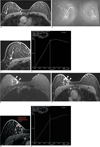
Fig. 4
Photographs of Sparganum obtained by surgical removal.
A. An ivory opaque cord-like tapeworm with a scolex (arrow) was extracted in the right breast upper inner quadrant. It measured 24.0×0.7 cm in size.
B. Photomicrograph of specimen (original magnification, ×100; hematoxylin-eosin [H-E] stain) show a sparganum larva (asterisk) with the characteristic pattern of noncellular tegument, cellular subtegument, and parenchyma bearing numerous bundles of muscle fibers and excretory canals. A surrounding foreign body granulomatous inflammation and inflammatory cell infiltration such as eosinophils, lymphocytes and histiocytes (arrowheads) are noted. The final pathologic diagnosis was breast sparganosis in the upper inner quadrant of the right breast.

References
1. Kim HS, Cha ES, Kim HH, Yoo JY. Spectrum of sonographic findings in superficial breast masses. J Ultrasound Med. 2005; 24:663–680.
2. Cho JH, Lee KB, Yong TS, Kim BS, Park HB, Ryu KN, et al. Subcutaneous and musculoskeletal sparganosis: imaging characteristics and pathologic correlation. Skeletal Radiol. 2000; 29:402–408.
3. Park JH, Chai JW, Cho NR, Paek NS, Guk SM, Shin EH, et al. A surgically confirmed case of breast sparganosis showing characteristic mammography and ultrasonography findings. Korean J Parasitol. 2006; 44:151–156.
4. Kim JE, Kim YJ, Kim MY, Han JY. A case of breast sparganosis: MR findings and ultrasonographic findings. Eur J Radiol Extra. 2007; 64:63–65.
5. Park HJ, Park NH, Lee EJ, Park CS, Lee SM, Park SI. Ultrasonographic findings of subcutaneous and muscular sparganosis. J Korean Soc Radiol. 2009; 61:183–187.
6. Jeong JK, Ryu BY, Lee HW, Kim HK, Choi CS. Sparganosis of the breast. J Korean Surg Soc. 1995; 48:428–432.
7. Chung SY, Park KS, Lee Y, Park CK. Breast sparganosis: mammographic and ultrasound features. J Clin Ultrasound. 1995; 23:447–451.
8. Moon HG, Jung EJ, Park ST. Breast sparganosis presenting as a breast mass with vague migrating pain. J Am Coll Surg. 2008; 207:292.
9. Kim YS, Hwang MS, Lee JK, Kim DS, Lee SK. US findings of breast sparganosis. J Korean Soc Med Ultrasound. 2003; 22:151–156.
10. Macura KJ, Ouwerkerk R, Jacobs MA, Bluemke DA. Patterns of enhancement on breast MR images: interpretation and imaging pitfalls. Radiographics. 2006; 26:1719–1734.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download