CASE DESCRIPTION
A 65-year-old Korean man had been diagnosed with pulmonary tuberculosis (TB) 40 years earlier. Four years before he presented to our clinic, he was told that he had TB-destroyed lung and a fungus ball. The patient visited our hospital on June 14, 2014 with dyspnea, blood-tinged sputum, and weight loss, which developed 2 months earlier. He denied experiencing fever, chills, or night sweats. He denied any history of overseas travel.
His initial blood pressure was 99/65 mmHg, respiratory rate was 16/min, and body temperature was 36.8°C. His chest was clear to auscultation. The laboratory results were as follows: hemoglobin, 11.3 g/dL; white blood cell count, 6,050/µL (64.7% neutrophils, 16.0% lymphocytes); platelet count, 291,000/µL; C-reactive protein, 5.56 mg/dL; blood urea nitrogen, 18 mg/dL; creatinine, 1.20 mg/dL; aspartate aminotransferase, 18 IU/L; alanine aminotransferase, 15 IU/L; and total bilirubin, 0.4 mg/dL.
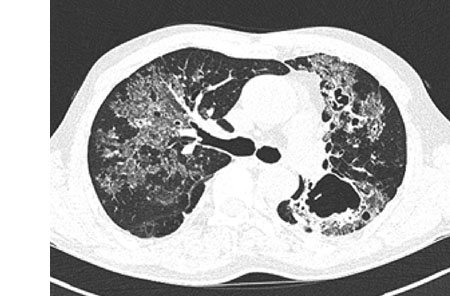
Blood and sputum cultures were negative for any pyogenic bacteria. Sputum was negative for acid-fast bacilli (AFB) by smear. A computed tomography (CT) scan of the chest showed numerous clumps of tiny nodules in both lungs, which were aggravated by the combination of bronchiectasis and cavitary lesions in both upper lobes and a fungus ball in the left upper lobe (
Fig. 1). No enlarged mediastinal or hilar lymph nodes were found. He had experienced a progressive decline in lung function and had recently developed recurrent pneumothorax.
Fig. 1
Initial and 4-month follow-up images of chest CT. (A, B) Initial chest CT showed numerous clumps of tiny nodules in both lungs are seen combined with bronchiectasis and cavitary lesions. (C, D) After 4 months, chest CT showed improvement of ground glass opacity and size of the lung lesion.
CT = computed tomography.
Serological and antigen tests for any fungus were negative. Culture studies of bronchial alveolar lavage fluid (BALF) were negative for any fungus. Antigens for histoplasmosis in the BALF were not investigated.
The patient underwent a VATS-based lung biopsy. The biopsy showed necrotizing granuloma and peribronchial lymphocytic infiltrations, and Gomori methenamine silver (GMS) staining revealed a fungal organism. AFB staining results were negative. The tissues contained multiple small (< 5 µm) yeast-like fungi. Considering the size and characteristics of the lesion, we suspected that these organisms were
Histoplasma spp. (
Fig. 2), and tissue culture confirmed
Histoplasma capsulatum. The patient was diagnosed with pulmonary histoplasmosis. Therapy was initiated with 200 mg itraconazole orally once per day for 12 months. He adhered to the treatment, and the symptoms disappeared 1 week after the treatment started. After 4 months, low-dose chest CT showed improvement of the ground glass opacity and size of the lung lesion (
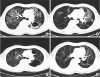
Fig. 1) although cystic lesions were increased. At the end of treatment, plain chest X-ray showed that the ground glass opacity lesion has almost disappeared (
Fig. 3).
Fig. 2
Histopathological examination of the lung. (A) Hematoxylin and eosin stain (× 40). (B) Acid-fast stain (× 100). (C) Periodic acid-Schiff stain (× 200). (D) Gomori methenamine silver stain (× 200). Many budding yeast-form fungal microorganisms can be seen.
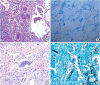
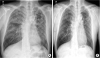
Fig. 3
Initial and 1 year after end of treatment images of CXR. (A) Initial CXR showed ground glass and reticular opacity in the both lungs. (B) No abnormal lesions were observed in CXR 1 year after end of treatment.
CXR = chest X-ray.
DISCUSSION
Histoplasmosis is the most common endemic mycosis in North America, Central America, and many countries in South America, and it also occurs in China, India, Southeast Asia, Africa, Australia, and Europe.
2 With the appearance of histoplasmosis in patients with acquired immunodeficiency syndrome, the endemic pattern has become more global.
3 The mold form of the organism (
H. capsulatum) grows in soil that has been enriched with bird or bat droppings, and it infects humans when the soil is disturbed and the microconidia are dispersed into the air.
4 Careful history taking that focused on areas visited in an endemic area or activities involving bats or birds, whether recent or remote, should aid in the differential diagnosis.
5
Our case is particularly interesting because Korea is not an endemic area, and the patient was immunocompetent and had no exposure history of travel abroad. Four cases of histoplasmosis in Korea have been reported to date. Two cases were associated with overseas travel history.
67 The third case involved an immunocompromised patient who had taken high-dose steroids for 6 months.
8 The fourth case was a newborn.
9 Our patient had no history of overseas travel or immunosuppression. This may be the first reported adult case of histoplasmosis in Korea that was not associated with immunosuppressed status and the third case without overseas travel history.
Clinical symptoms of histoplasmosis range from asymptomatic infection to diffuse alveolar disease, which may cause respiratory difficulty and even death. The extent of the disease and the mode of presentation following initial exposure correlate with the size of the inhaled inoculum and the
Histoplasma-specific immunity of the exposed person. After heavy exposure, nonimmune people usually present with respiratory symptoms (fever, myalgia, dry cough, and dyspnea) within 2 weeks and diffuse pulmonary involvement on radiographic assessment. However, with low-level exposure, pulmonary illness is more commonly subacute and mild, and may even be asymptomatic.
4
Pulmonary histoplasmosis is classified into acute, subacute, and chronic pulmonary histoplasmosis, based on the duration, radiologic findings, and underlying lung infection.
3 Our patient had chronic pulmonary histoplasmosis in terms of the duration of his symptoms, CT findings, and underlying TB-destroyed lung. The diagnosis of histoplasmosis may differ according to the clinical situation. Laboratory diagnosis involves a mycological test, culture, fungus staining, antigen detection, and serological tests for antibodies. Each of these tests plays a useful role but has important limitations in some conditions.
1011 First, serological tests for antibodies may remain positive for several years after the infection with
H. capsulatum, complicating the interpretation of test results in patients with atypical illness. In asymptomatic histoplasmosis presenting with a solitary pulmonary nodule, antigen detection and serological tests are less sensitive than tests in symptomatic histoplasmosis patients. Second, the greatest fungal culture positivity occurs in those patients who have disseminated infection and acute pulmonary histoplasmosis after exposure to a large inoculum of the organism, but fungal culture is less sensitive in cases with a low inoculum of the organism.
12 In addition, cultures may take up to 6 weeks to grow, which may delay the diagnosis if only this method is used.
3
Histoplasma antigen may be detected in BALF and urine in up to 75% of cases with acute and diffuse infiltration.
31314 In patients with chronic pulmonary histoplasmosis, such as our patient, tests for antigen in the urine and blood are usually negative because of the low fungal burden.
1516 However, if bronchoscopy is performed, bronchial washings or BALF can be tested for antigen, which may reduce the time for diagnosis. Thus, if the clinical findings are atypical for histoplasmosis and suggestive of malignancy, a biopsy may be required to differentiate these conditions.
Histopathological identification of the typical 2–4-µm budding yeasts in tissue biopsies is a rapid means of identifying a patient with histoplasmosis.
1217 Staining the tissue with GMS or periodic acid-Schiff stain is necessary to visualize the small yeast structures.
18
The Infectious Disease Society of America has published detailed treatment guidelines,
1 which detail the various treatment options available. The first decision to be made is whether to treat or to observe. In asymptomatic patients, treatment is not indicated. However, symptomatic patients with diffuse infiltrative lesions should be treated with amphotericin B at a dose of 0.7 mg/kg/day (or a lipid preparation at a dose of 3 mg/kg/day for patients with renal impairment). After discharge, itraconazole at a dose of 200 mg once or twice daily should be given for 12 weeks. In chronic pulmonary histoplasmosis, such as in our case, the clinical and radiographic findings resemble those seen in reactivation TB. Without treatment, the illness is progressive and can cause loss of pulmonary function in most patients and death in up to half of all patients. Thus, treatment is indicated in all patients with chronic pulmonary histoplasmosis; itraconazole at a dose of 200 mg once or twice daily for 12–24 months is the treatment of choice for chronic pulmonary histoplasmosis.
1
In conclusion, we report on a case involving an immunocompetent patient who developed histoplasmosis. We suggest two lessons to learn from our case. First, patients with histoplasmosis were reported several times in Korea not known as an endemic area. Therefore, we should consider all possibility including histoplasmosis in patients with non-specific respiratory symptoms and progressive lung destruction. Second, physicians should consider using an assertive method for diagnosis, such as VATS biopsy, in cases with atypical findings and unexplainable progressive infiltrative lung lesions.